Graham-Little-Piccardi-Lasseur Syndrome: A Case Report
173811738117381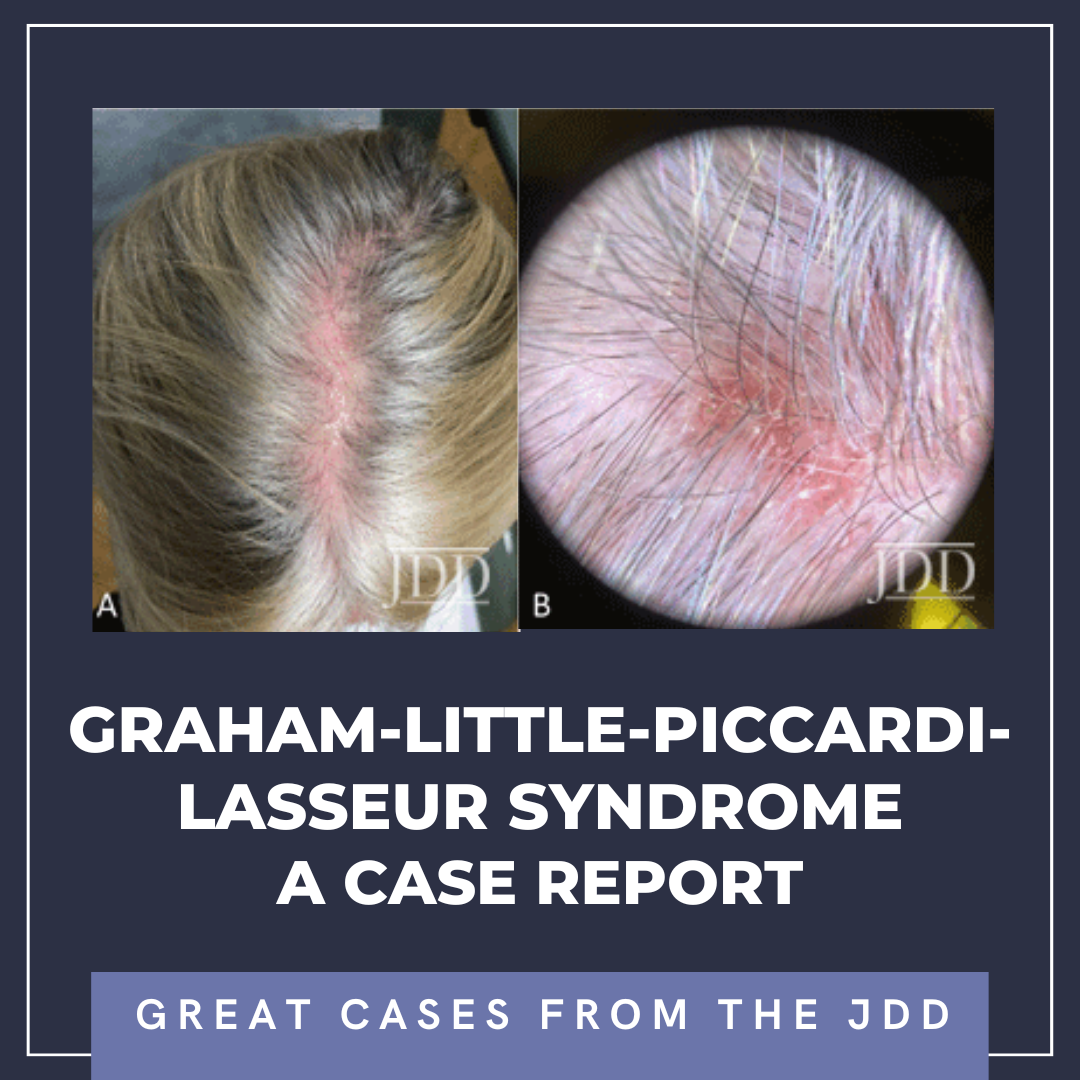 INTRODUCTION
Graham-Little-Piccardi-Lasseur Syndrome (GLPLS) is a rare clinical subtype of lichen planopilaris (LPP) that manifests as a triad of scarring alopecia of the scalp, nonscarring alopecia of the axillary and the pubic skin, and widespread lichenoid follicular papules.1 GLPLS more commonly affects women (male-to-female ratio ≃ 1:4), with the classic patient being a middle-aged Ca …
INTRODUCTION
Graham-Little-Piccardi-Lasseur Syndrome (GLPLS) is a rare clinical subtype of lichen planopilaris (LPP) that manifests as a triad of scarring alopecia of the scalp, nonscarring alopecia of the axillary and the pubic skin, and widespread lichenoid follicular papules.1 GLPLS more commonly affects women (male-to-female ratio ≃ 1:4), with the classic patient being a middle-aged Ca …
 INTRODUCTION
Graham-Little-Piccardi-Lasseur Syndrome (GLPLS) is a rare clinical subtype of lichen planopilaris (LPP) that manifests as a triad of scarring alopecia of the scalp, nonscarring alopecia of the axillary and the pubic skin, and widespread lichenoid follicular papules.1 GLPLS more commonly affects women (male-to-female ratio ≃ 1:4), with the classic patient being a middle-aged Ca …
INTRODUCTION
Graham-Little-Piccardi-Lasseur Syndrome (GLPLS) is a rare clinical subtype of lichen planopilaris (LPP) that manifests as a triad of scarring alopecia of the scalp, nonscarring alopecia of the axillary and the pubic skin, and widespread lichenoid follicular papules.1 GLPLS more commonly affects women (male-to-female ratio ≃ 1:4), with the classic patient being a middle-aged Ca … Continue reading "Graham-Little-Piccardi-Lasseur Syndrome: A Case Report"


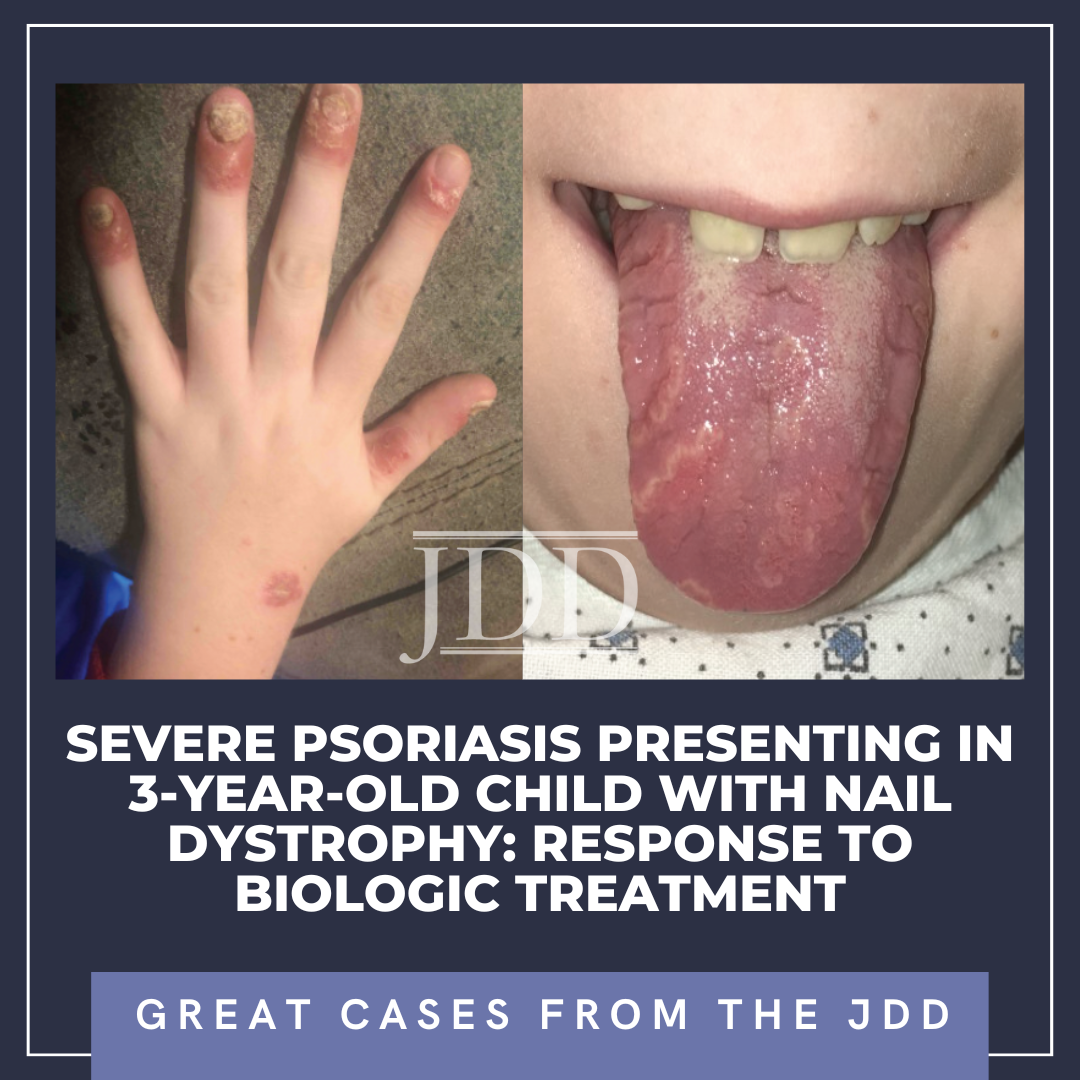 Severe Psoriasis Presenting in 3-Year-Old Child With Nail Dystrophy: Response to Biologic Treatment
Danielle Rinck MDa, Elaine Siegfried MDb
aBeth Israel Deaconess Medical Center, Boston, MA | bSaint Louis University School of Medicine, St. Louis, MO
J Drugs Dermatol. 2022;21(8):897-899. doi:10.36849/JDD.6888
A previously healthy 3-year-old boy presented to our Pediatric Derm …
Severe Psoriasis Presenting in 3-Year-Old Child With Nail Dystrophy: Response to Biologic Treatment
Danielle Rinck MDa, Elaine Siegfried MDb
aBeth Israel Deaconess Medical Center, Boston, MA | bSaint Louis University School of Medicine, St. Louis, MO
J Drugs Dermatol. 2022;21(8):897-899. doi:10.36849/JDD.6888
A previously healthy 3-year-old boy presented to our Pediatric Derm … 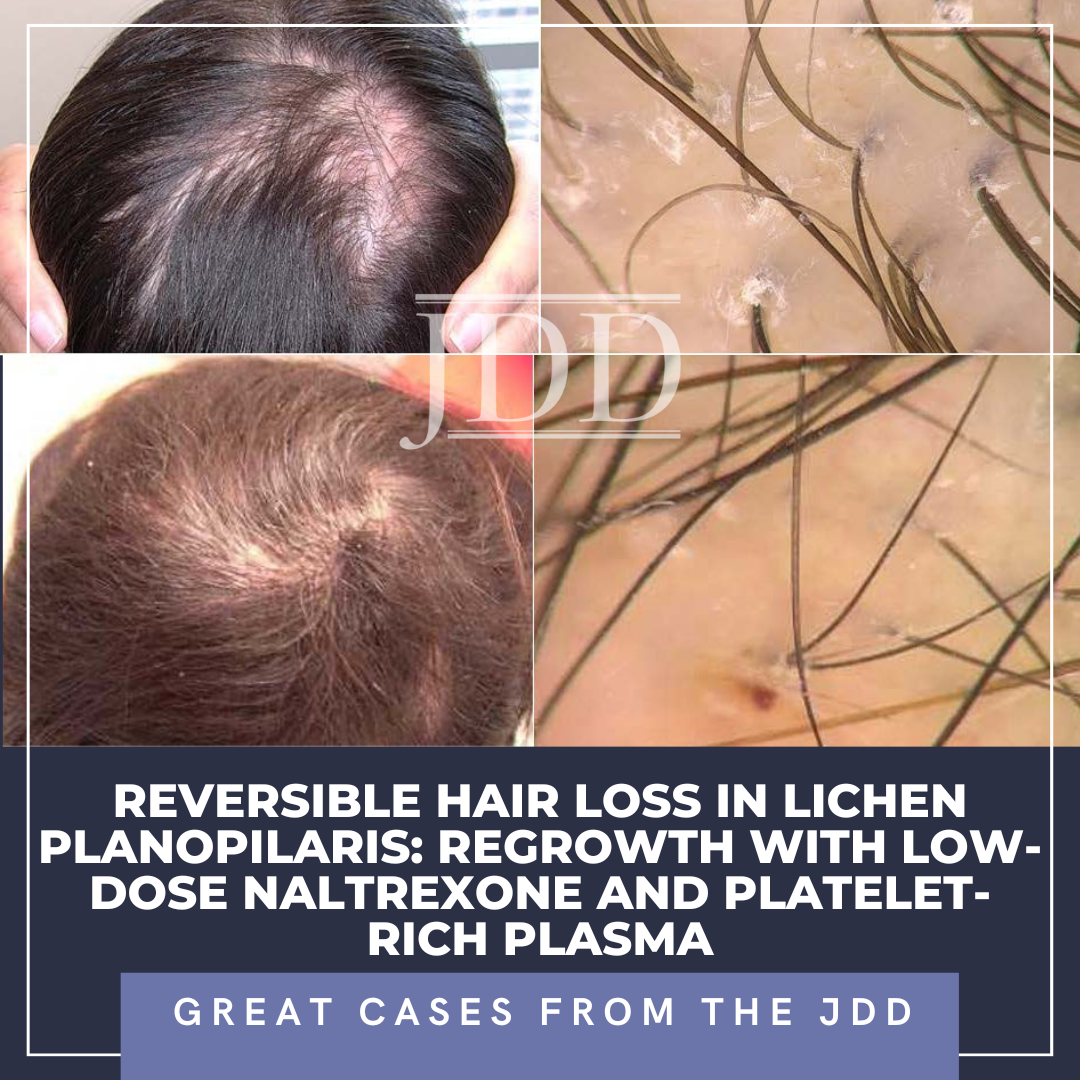 INTRODUCTION
Lichen planopilaris (LPP) is a cicatricial alopecia that presents with patchy or diffuse hair loss at the vertex or parietal scalp. Although there is no gold standard therapy, most interventions are immune modulating and aimed at reducing inflammation and terminating the scarring process to prevent further fibrosis.3 Even amongst patients who respond to therapy, hair loss at alopeci …
INTRODUCTION
Lichen planopilaris (LPP) is a cicatricial alopecia that presents with patchy or diffuse hair loss at the vertex or parietal scalp. Although there is no gold standard therapy, most interventions are immune modulating and aimed at reducing inflammation and terminating the scarring process to prevent further fibrosis.3 Even amongst patients who respond to therapy, hair loss at alopeci … 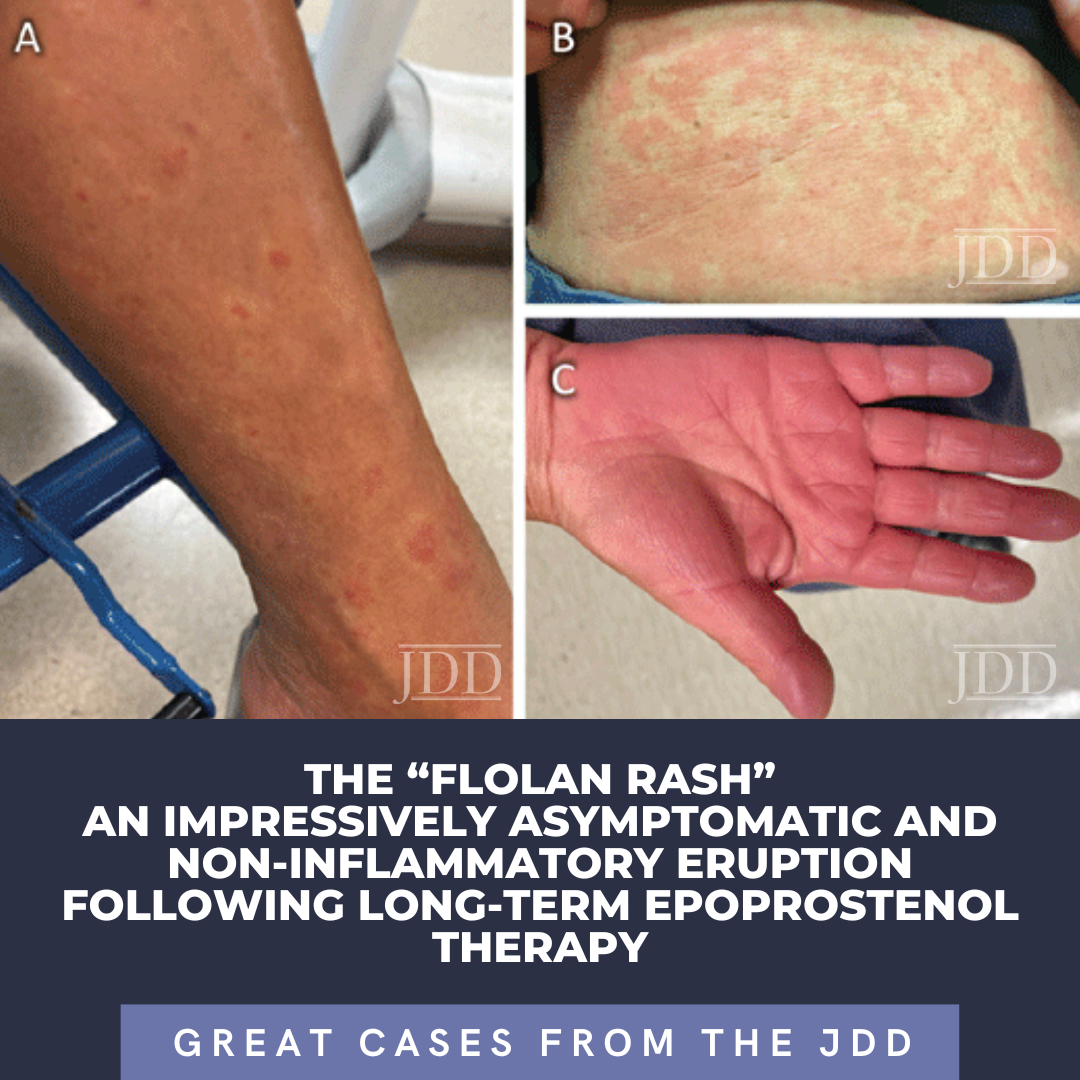 INTRODUCTION
Epoprostenol (Flolan) is a last-resort intravenous (IV) medication for the treatment of severe pulmonary arterial hypertension (PAH). Cutaneous adverse events of Flolan are well-known by pulmonologists, though lacking in dermatologic literature.1 We report an extensive near erythrodermic appearing asymptomatic eruption following long-term use of epoprostenol. This characteristic and …
INTRODUCTION
Epoprostenol (Flolan) is a last-resort intravenous (IV) medication for the treatment of severe pulmonary arterial hypertension (PAH). Cutaneous adverse events of Flolan are well-known by pulmonologists, though lacking in dermatologic literature.1 We report an extensive near erythrodermic appearing asymptomatic eruption following long-term use of epoprostenol. This characteristic and … 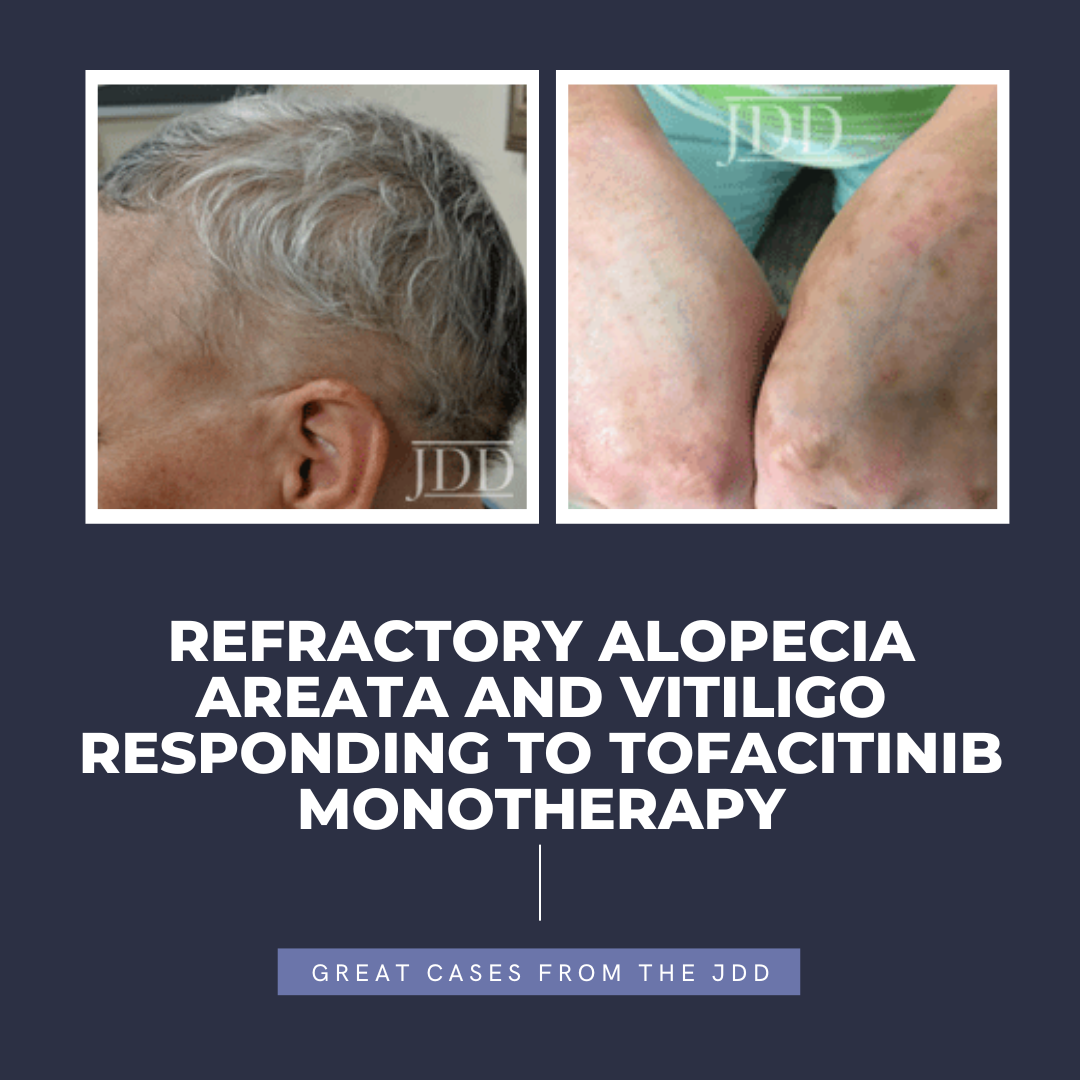 INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …
INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …