Dermal Hypersensitivity Reaction to Semaglutide: Two Case Reports
175251752517525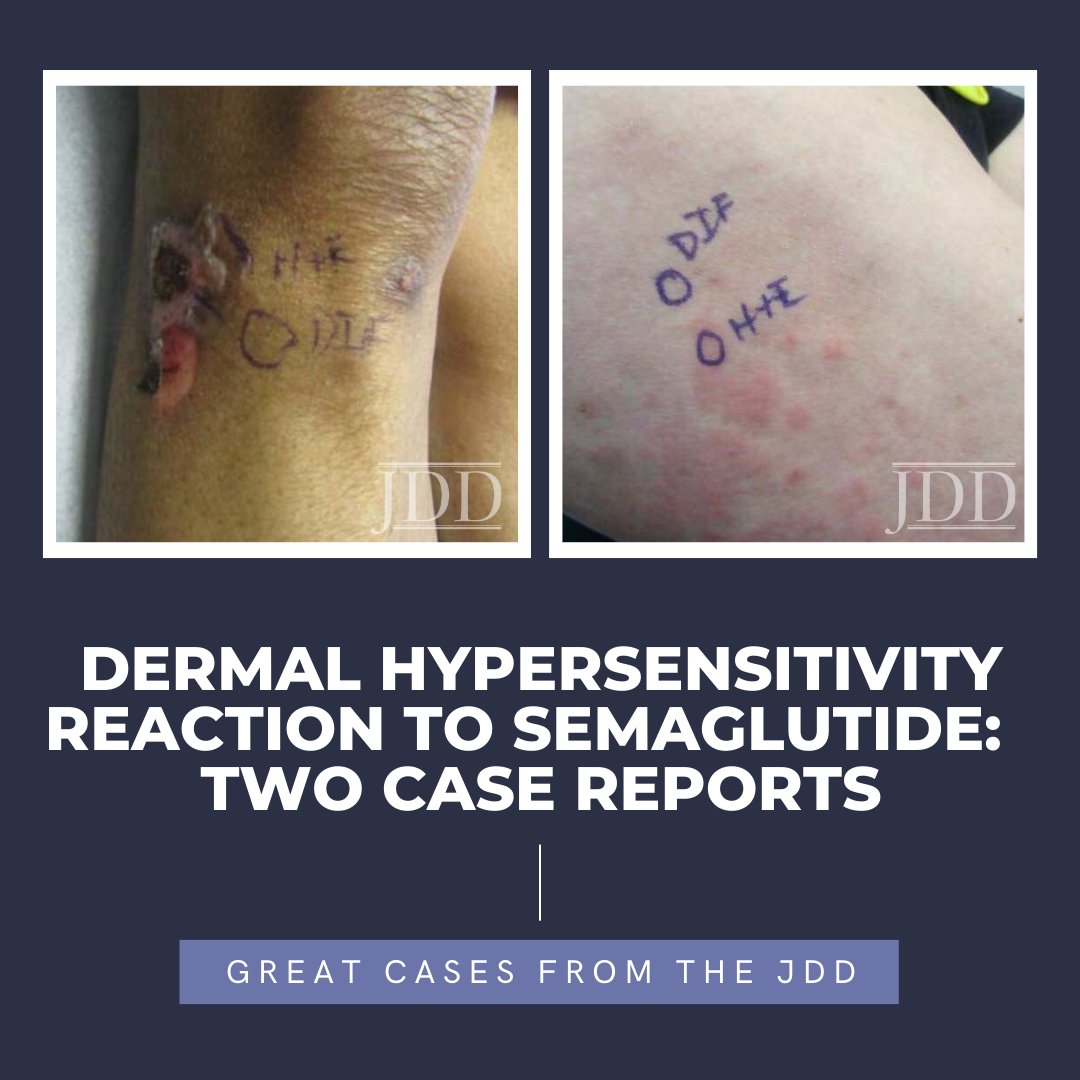 Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to …
Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to …
 Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to …
Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to … Continue reading "Dermal Hypersensitivity Reaction to Semaglutide: Two Case Reports"


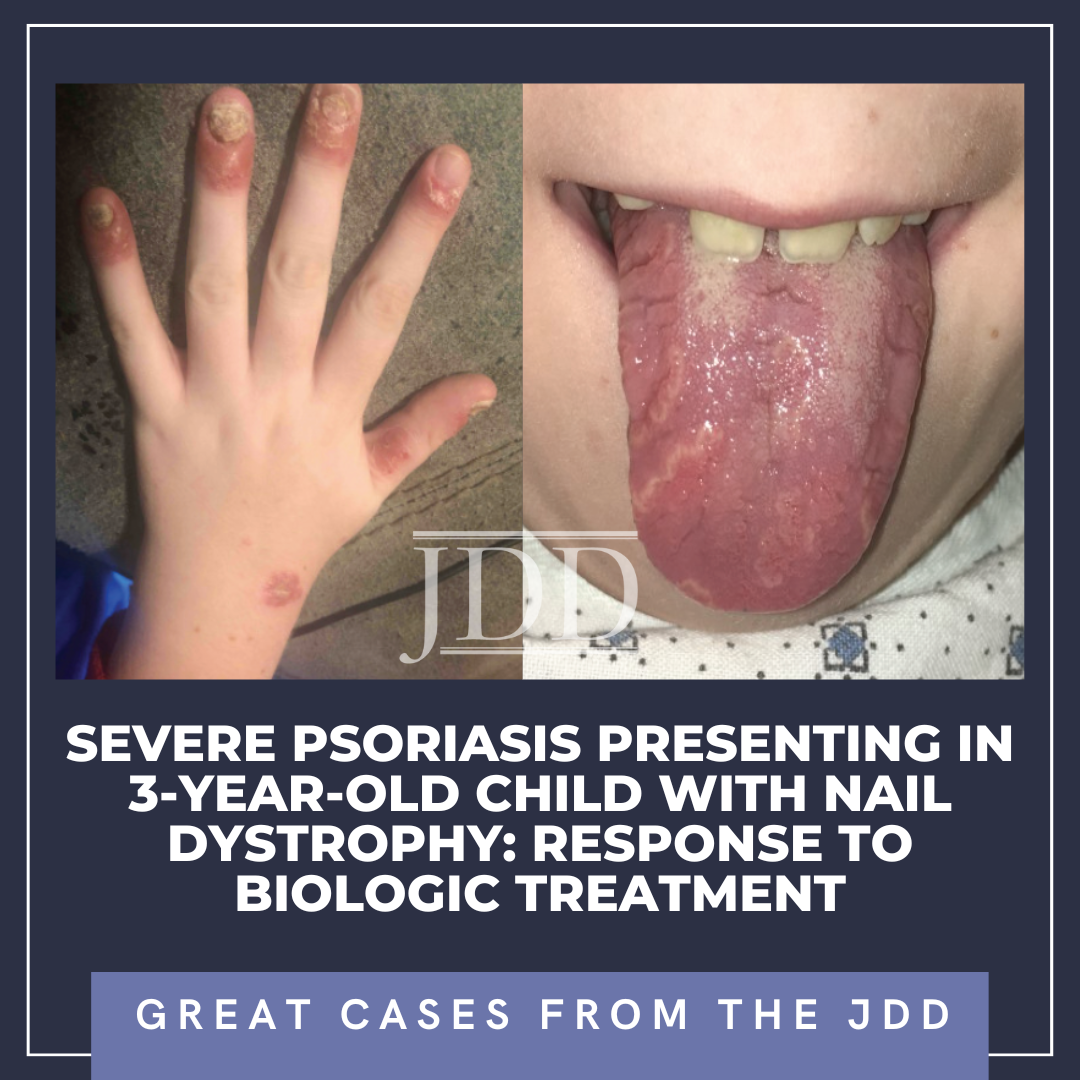 Severe Psoriasis Presenting in 3-Year-Old Child With Nail Dystrophy: Response to Biologic Treatment
Danielle Rinck MDa, Elaine Siegfried MDb
aBeth Israel Deaconess Medical Center, Boston, MA | bSaint Louis University School of Medicine, St. Louis, MO
J Drugs Dermatol. 2022;21(8):897-899. doi:10.36849/JDD.6888
A previously healthy 3-year-old boy presented to our Pediatric Derm …
Severe Psoriasis Presenting in 3-Year-Old Child With Nail Dystrophy: Response to Biologic Treatment
Danielle Rinck MDa, Elaine Siegfried MDb
aBeth Israel Deaconess Medical Center, Boston, MA | bSaint Louis University School of Medicine, St. Louis, MO
J Drugs Dermatol. 2022;21(8):897-899. doi:10.36849/JDD.6888
A previously healthy 3-year-old boy presented to our Pediatric Derm … 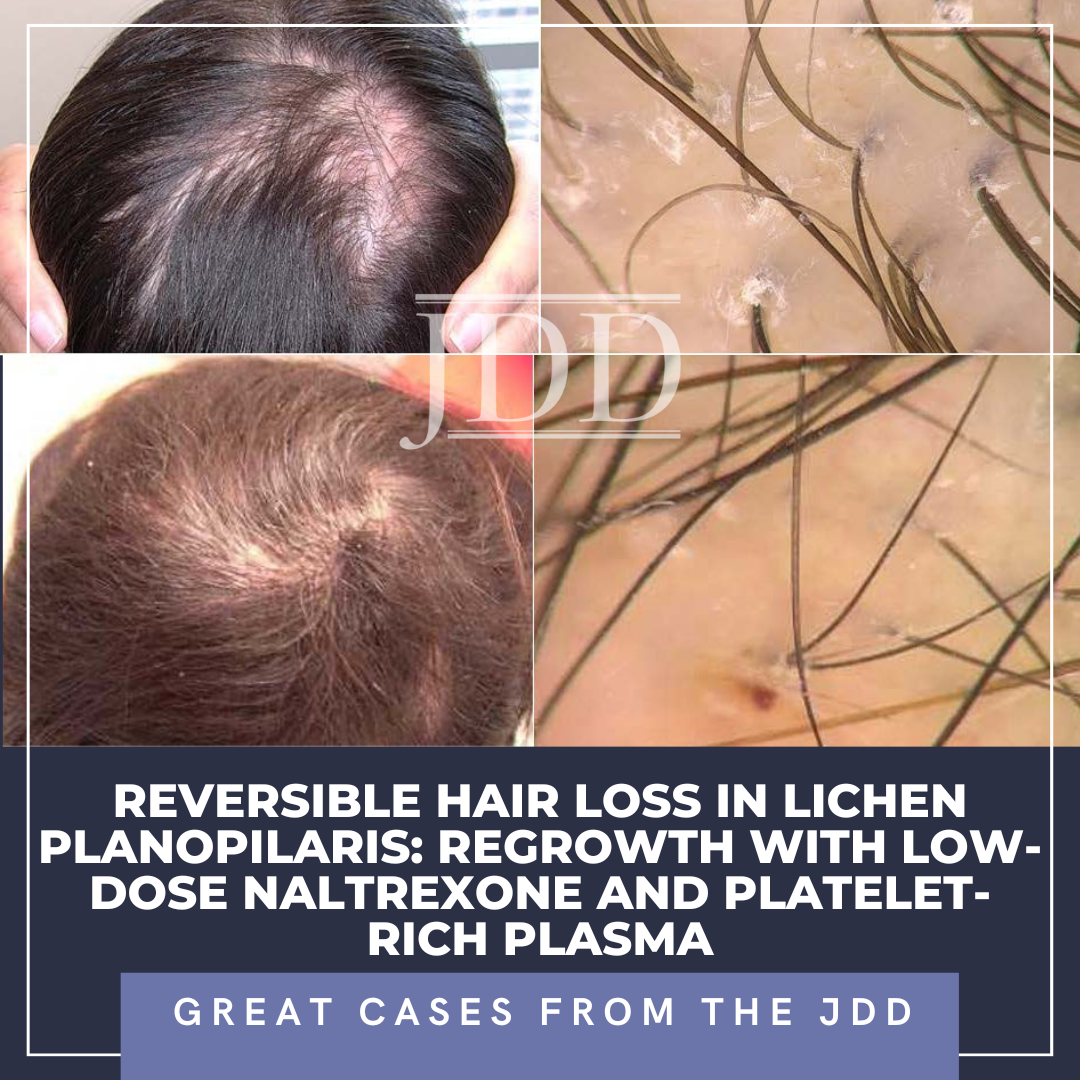 INTRODUCTION
Lichen planopilaris (LPP) is a cicatricial alopecia that presents with patchy or diffuse hair loss at the vertex or parietal scalp. Although there is no gold standard therapy, most interventions are immune modulating and aimed at reducing inflammation and terminating the scarring process to prevent further fibrosis.3 Even amongst patients who respond to therapy, hair loss at alopeci …
INTRODUCTION
Lichen planopilaris (LPP) is a cicatricial alopecia that presents with patchy or diffuse hair loss at the vertex or parietal scalp. Although there is no gold standard therapy, most interventions are immune modulating and aimed at reducing inflammation and terminating the scarring process to prevent further fibrosis.3 Even amongst patients who respond to therapy, hair loss at alopeci … 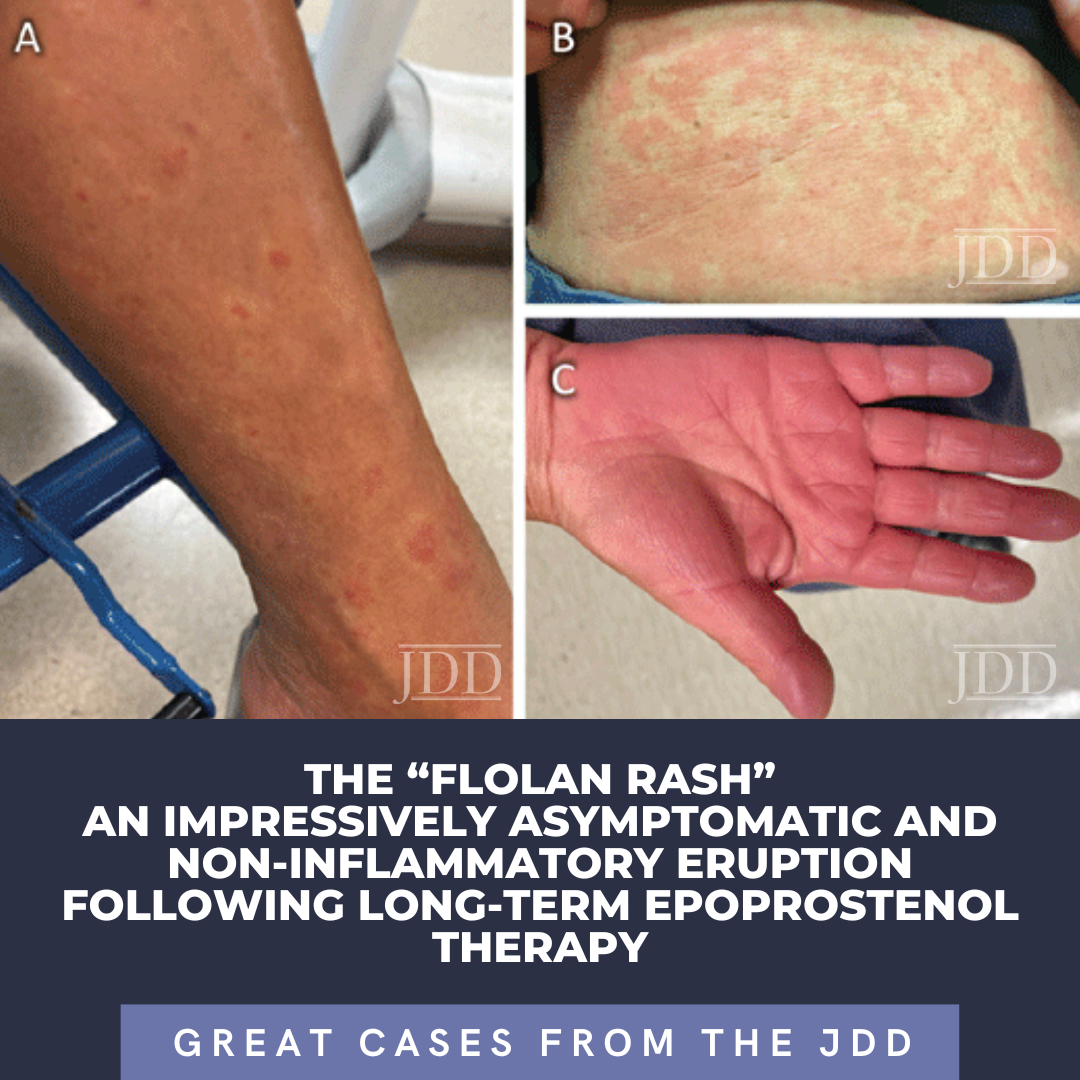 INTRODUCTION
Epoprostenol (Flolan) is a last-resort intravenous (IV) medication for the treatment of severe pulmonary arterial hypertension (PAH). Cutaneous adverse events of Flolan are well-known by pulmonologists, though lacking in dermatologic literature.1 We report an extensive near erythrodermic appearing asymptomatic eruption following long-term use of epoprostenol. This characteristic and …
INTRODUCTION
Epoprostenol (Flolan) is a last-resort intravenous (IV) medication for the treatment of severe pulmonary arterial hypertension (PAH). Cutaneous adverse events of Flolan are well-known by pulmonologists, though lacking in dermatologic literature.1 We report an extensive near erythrodermic appearing asymptomatic eruption following long-term use of epoprostenol. This characteristic and … 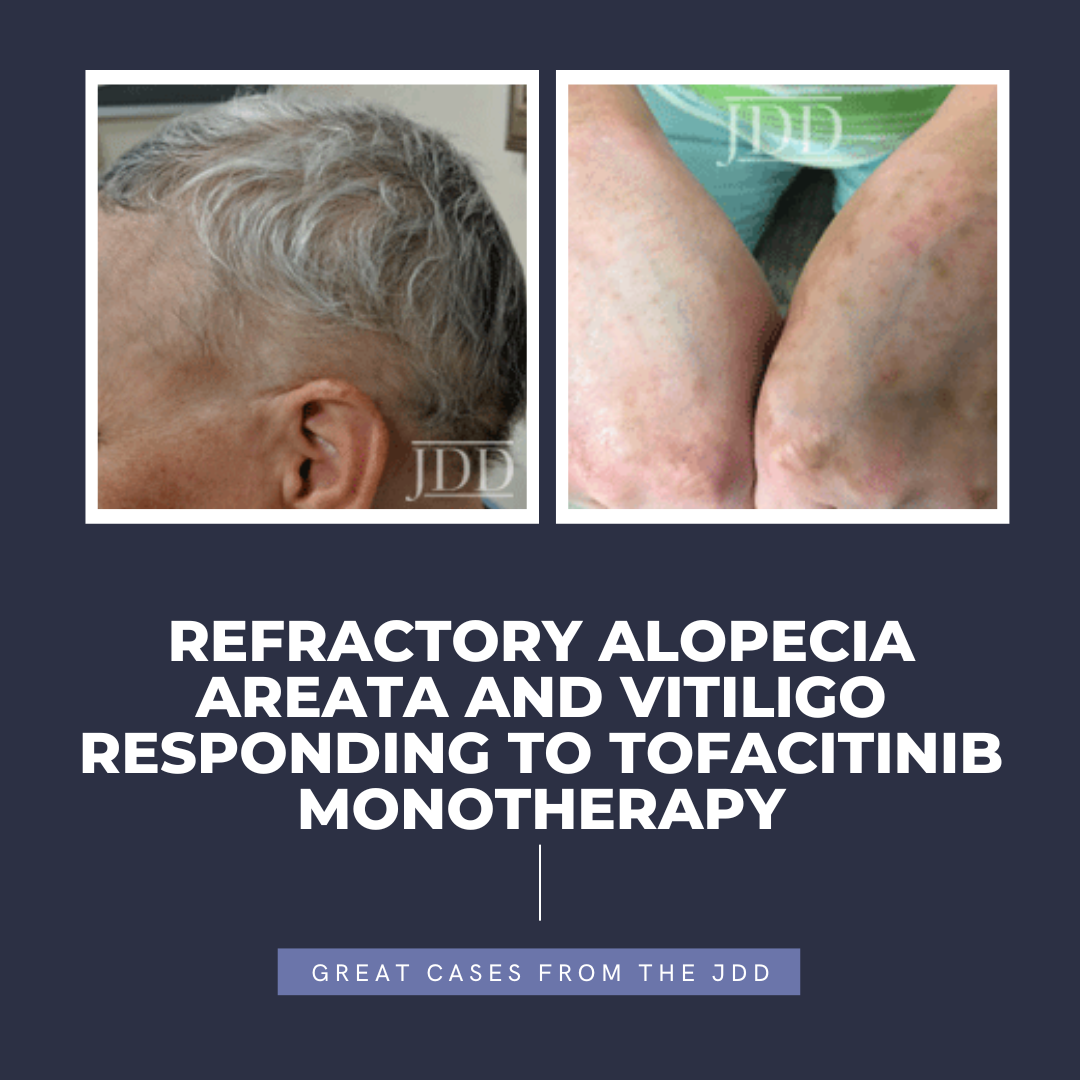 INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …
INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib …