JDD June 2023 Issue Highlights
176051760517605 The June issue of the Journal of Drugs in Dermatology (JDD) features original articles with topics ranging from pediatric acne, rosacea, androgenetic alopecia, precision medicine, infantile hemangiomas, and many more. Check out this month’s issue highlights straight from the JDD Editor’s desk:
The Many Faces of Pediatric Acne: A Practical Algorithm for Treatment, Maintenance Therapy, and …
The June issue of the Journal of Drugs in Dermatology (JDD) features original articles with topics ranging from pediatric acne, rosacea, androgenetic alopecia, precision medicine, infantile hemangiomas, and many more. Check out this month’s issue highlights straight from the JDD Editor’s desk:
The Many Faces of Pediatric Acne: A Practical Algorithm for Treatment, Maintenance Therapy, and …
 The June issue of the Journal of Drugs in Dermatology (JDD) features original articles with topics ranging from pediatric acne, rosacea, androgenetic alopecia, precision medicine, infantile hemangiomas, and many more. Check out this month’s issue highlights straight from the JDD Editor’s desk:
The Many Faces of Pediatric Acne: A Practical Algorithm for Treatment, Maintenance Therapy, and …
The June issue of the Journal of Drugs in Dermatology (JDD) features original articles with topics ranging from pediatric acne, rosacea, androgenetic alopecia, precision medicine, infantile hemangiomas, and many more. Check out this month’s issue highlights straight from the JDD Editor’s desk:
The Many Faces of Pediatric Acne: A Practical Algorithm for Treatment, Maintenance Therapy, and … 

 Next Steps in Derm and the Journal of Drugs in Dermatology, in partnership with the Dermatology Education Foundation (DEF) and Physicians Resources, interviewed Dr. Hilary Baldwin, a board-certified dermatologist and medical director of the Acne Treatment & Research Center in Morristown, NJ and Brooklyn, NY. With the heavy acne tool box these days, how do you pick which medications to use? …
Next Steps in Derm and the Journal of Drugs in Dermatology, in partnership with the Dermatology Education Foundation (DEF) and Physicians Resources, interviewed Dr. Hilary Baldwin, a board-certified dermatologist and medical director of the Acne Treatment & Research Center in Morristown, NJ and Brooklyn, NY. With the heavy acne tool box these days, how do you pick which medications to use? … 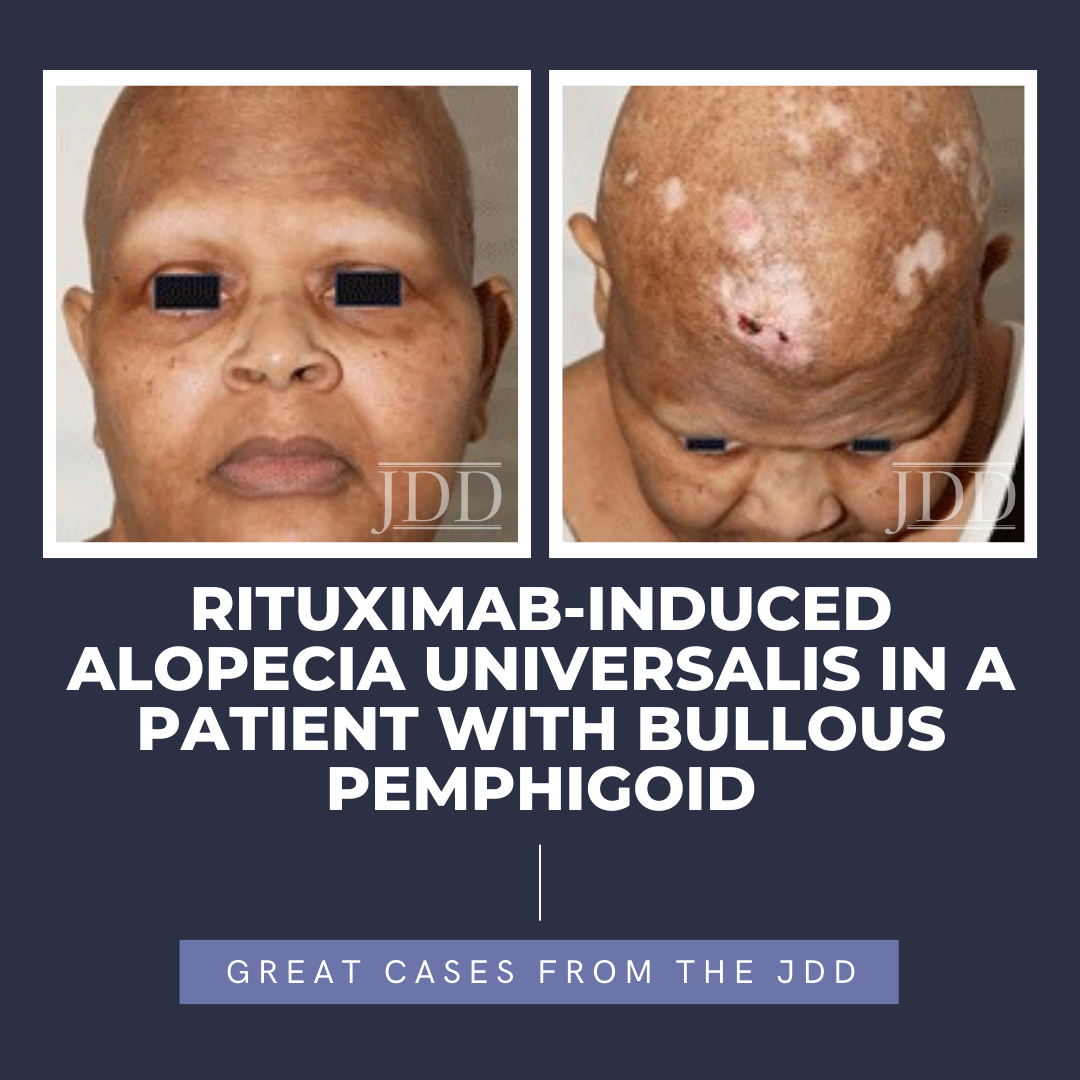 Alopecia areata is a CD8+ T-lymphocyte driven autoimmune disorder leading to reversible hair loss. While most commonly presenting as isolated well-demarcated non-cicatricial alopecic patches on the scalp, subtypes of alopecia areata include alopecia totalis with loss of all scalp hair and alopecia universalis with complete loss of all body hair. Although primarily an idiopathic condition, several …
Alopecia areata is a CD8+ T-lymphocyte driven autoimmune disorder leading to reversible hair loss. While most commonly presenting as isolated well-demarcated non-cicatricial alopecic patches on the scalp, subtypes of alopecia areata include alopecia totalis with loss of all scalp hair and alopecia universalis with complete loss of all body hair. Although primarily an idiopathic condition, several …  Next Steps in Derm and the Journal of Drugs in Dermatology, in partnership with the Dermatology Education Foundation (DEF) and Physicians Resources, interviewed Dr. Mark Gimbel, surgical oncologist with Banner MD Anderson Cancer Center, on how molecular tools are revolutionizing how melanoma is diagnosed, treated and prognosticated. Watch Dr. Gimbel explain the current tests on the market and …
Next Steps in Derm and the Journal of Drugs in Dermatology, in partnership with the Dermatology Education Foundation (DEF) and Physicians Resources, interviewed Dr. Mark Gimbel, surgical oncologist with Banner MD Anderson Cancer Center, on how molecular tools are revolutionizing how melanoma is diagnosed, treated and prognosticated. Watch Dr. Gimbel explain the current tests on the market and … 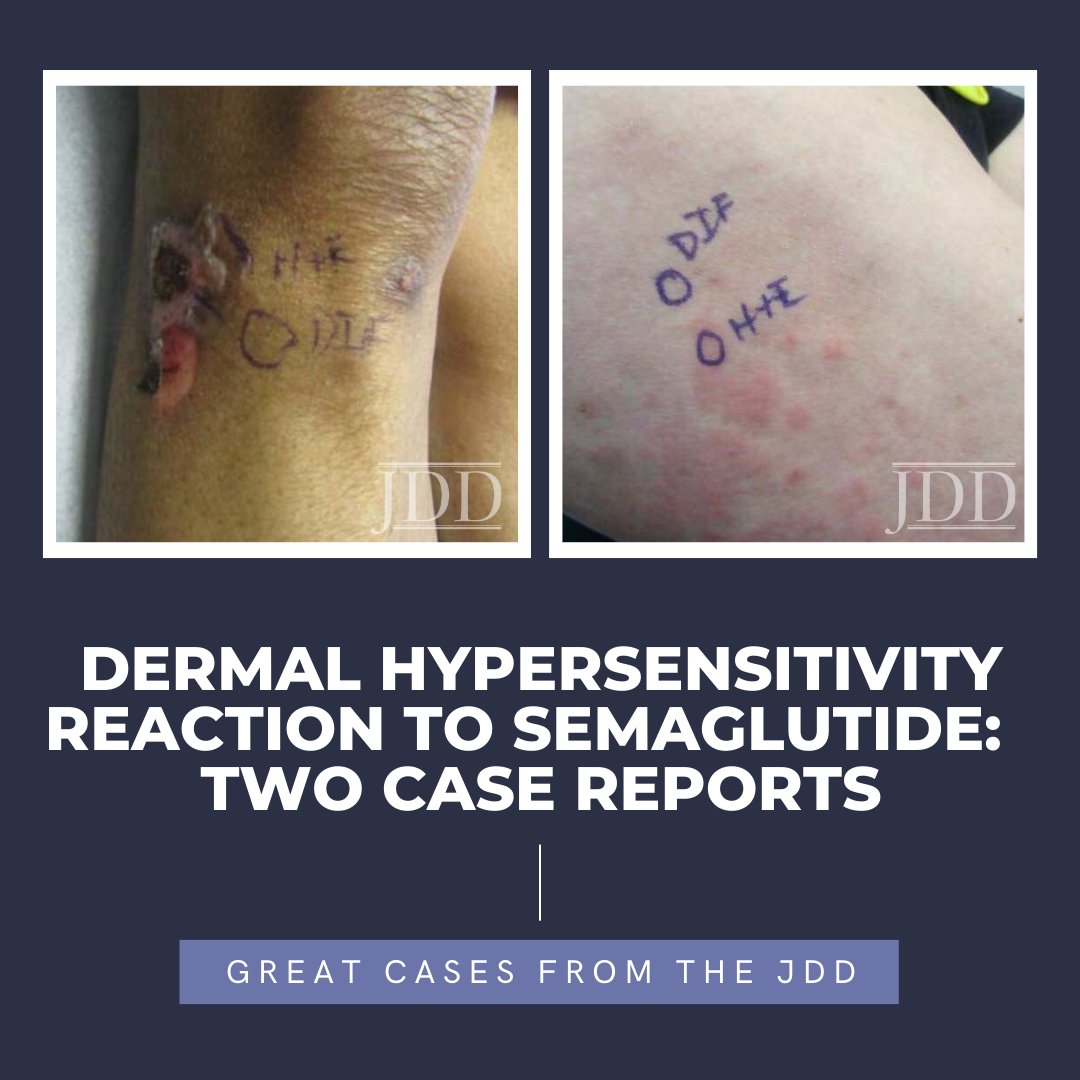 Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to …
Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that was FDA-approved in 2017 for treatment of type II diabetes and in 2021 for treatment for chronic weight management in adults with obesity or overweight with at least one weight-related condition.1 Due to its longer duration of action, it is typically administered subcutaneously once weekly. The safety profile of semaglutide is similar to …