This is the first edition of a quarterly column highlighting articles of interest to dermatologists from non-dermatologic literature. It is already difficult to keep up to date with the high volume and forever expanding literature in dermatologic journals. Additionally, it is virtually impossible to read the full breadth of biomedical literature for materials relevant to the field of dermatology. I created this column in an attempt to close the gap and highlight key literature relevant to our field published outside well-recognized dermatologic journals. If there is an article /study you come across from the non-dermatologic literature relevant to dermatology, please email me at [email protected].
Now onto what’s new in dermatology from the outside world…
Have you ever wondered:
-
- Does stress cause grey hair?
- Why does skin expand when you stretch it?
- Why do patients with Birt-Hogg-Dubé syndrome get renal tumors?
- What vaccine therapies are being developed to treat melanoma?
Read on to find out the answers to all the above!
Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells
True or False: Stress causes grey hair.
The notion that stress causes grey hair is a popular belief. Hair color is determined by melanocytes, which produce melanin to color the hair follicle as it grows.
 Zhang et al. established the link between stress and hair greying. The team demonstrated in mice that in response to stress the sympathetic nervous system over-activates. This causes the release of the neurotransmitter, norepinephrine, which causes premature activation of melanocytes stem cells. Following premature activation, these melanocyte stem cells are not replaced, leading to depletion of the hair’s “reserve” of pigment, aka color. So, new hair that regrows from hair follicles that have lost melanocyte stem cells has less pigment and appears gray.
Zhang et al. established the link between stress and hair greying. The team demonstrated in mice that in response to stress the sympathetic nervous system over-activates. This causes the release of the neurotransmitter, norepinephrine, which causes premature activation of melanocytes stem cells. Following premature activation, these melanocyte stem cells are not replaced, leading to depletion of the hair’s “reserve” of pigment, aka color. So, new hair that regrows from hair follicles that have lost melanocyte stem cells has less pigment and appears gray.
So if you feel like your kids, spouse or quarantine is making you greyer, you may be onto something!
Mechanisms of stretch-mediated skin expansion at single-cell resolution
 Skin is a highly dynamic organ and must adapt its shape and size to maintain vital barrier functions. It is highly anisotropic with larger stiffness along pronounced collagen fiber orientations, aka Langer’s lines. How does the skin maintain its integrity when chronically stretched beyond its physiological limit? It does so by increasing its surface area to reduce the mechanical load.
Skin is a highly dynamic organ and must adapt its shape and size to maintain vital barrier functions. It is highly anisotropic with larger stiffness along pronounced collagen fiber orientations, aka Langer’s lines. How does the skin maintain its integrity when chronically stretched beyond its physiological limit? It does so by increasing its surface area to reduce the mechanical load.
So, why does the skin grow bigger as you stretch it? The skin’s fascinating response to stretch is finally explained.
Aragona et al. provided convincing evidence of the intricate communication between mechanical strain and a subpopulation of cutaneous stem cells to make new tissue in mice. The authors believe that a similar mechanism may be mediating tissue expansion in humans, which has revolutionized reconstructive skin surgery for the treatment of burn injuries, birth defects, and breast reconstruction.
To be brief, the group demonstrated that in response to mechanical strain (via a self-inflating gel) the actomyosin cytoskeleton of a subpopulation basal layer stem cells is reorganized. This triggers translocation of transcription factors YAP, TAZ, and MAL to the nucleus mediating gene-expression changes that promote an increase in basal layer stem cell proliferation and differentiation. Together, these changes lead to skin tissue expansion while maintaining its protective function.
This begs the question, what exactly happens during a period of rapid weight gain, such as pregnancy? What happens to those stem cells? Localized stem cells apoptosis due to too much strain? Is the mechanical strain too acute, not providing enough time for the basal layer stem cells to mediate skin expansion to accommodate to reduce the mechanical load? Hence, why we get the stretch marks, we all love to hate. Looking forward to the elucidation of this mechanism too!
Further reading on this topic:
Growth on demand: reviewing the mechanobiology of stretched skin
A substrate-specific mTORC1 pathway underlies Birt-Hogg-Dubé syndrome
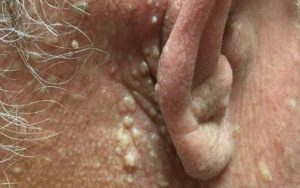 Birt-Hogg-Dubé syndrome (BHDS) is a rare autosomal dominant genodermatosis that affects the skin, lung, and kidney. It is caused by a germline loss of function mutation in the FLCN gene, which encodes the protein folliculin. Cutaneous manifestations include multiple fibrofolliculomas (most common), trichodiscomas, and acrochordon-like lesions. The major systemic manifestations are renal tumors, pulmonary cysts, and spontaneous pneumothoraces. Affected individuals have a 7-fold increased risk of renal tumors compared to the general population.
Birt-Hogg-Dubé syndrome (BHDS) is a rare autosomal dominant genodermatosis that affects the skin, lung, and kidney. It is caused by a germline loss of function mutation in the FLCN gene, which encodes the protein folliculin. Cutaneous manifestations include multiple fibrofolliculomas (most common), trichodiscomas, and acrochordon-like lesions. The major systemic manifestations are renal tumors, pulmonary cysts, and spontaneous pneumothoraces. Affected individuals have a 7-fold increased risk of renal tumors compared to the general population.
Writing in Nature, Napolitano et al. highlighted the mechanism critical to kidney tumorgenesis in BHDS. The authors demonstrated that loss of function of FLCN leads to nuclear translocation and constitutive activation of transcription factor EB (TFEB), a master regulator of lysosomal biogenesis and autophagy. Constitutively active TFEB mediates transcriptional induction and increased expression of RagC and RagD GTPases which leads to over-activity of the mechanistic target of rapamycin complex 1 (mTORC1), a master regulator of cell growth and proliferation. Importantly, when they knocked out TFEB in mice model of Birt-Hogg-Dubé syndrome, the mice developed normal kidney size, morphology, and histology, as well as normal kidney function. Together, their experiments elegantly identified TFEB activation as crucial to renal tumorigenesis in BHDS.
Given that prognosis of patients with BHDS mainly relates to the development of renal cancer, this underscores TFEB as a possible prognostic and/or therapeutic target in the management of patients with Birt-Hogg-Dubé syndrome or renal tumor in general.
An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma
 Cancer vaccines remain a promising approach for the management of melanoma, with only one FDA-approved vaccine (Talimogene Laherparepvec) against un-resectable melanoma. Nonetheless, the vaccine approach to melanoma treatment has not yet fully materialized.
Cancer vaccines remain a promising approach for the management of melanoma, with only one FDA-approved vaccine (Talimogene Laherparepvec) against un-resectable melanoma. Nonetheless, the vaccine approach to melanoma treatment has not yet fully materialized.
mRNA vaccines represent a promising alternative to conventional vaccine approaches due to their innate immunogenic properties. mRNA is a non-infectious, non-integrating platform, that gets degraded by normal cellular processes. mRNA vaccines are attractive because of their high potency, capacity for rapid development, and potential for low-cost manufacture and safe administration.
Sahin et al. are testing an mRNA-based vaccine, FixVac (BNT111) for the management of patients with stage IIIB, C and stage IV melanoma. FixVac is composed of RNA-LPX encoding four tumor-associated antigens and is administered intravenously. Data from this multicenter, open-label, phase I trial (Lipo-MERIT) are promising and demonstrated that FixVac is not only active as a single agent but synergizes with anti-PD1 therapy in patients treated with checkpoint inhibitors. Their observation highlights the possible role of mRNA-based vaccines in combination treatment with checkpoint inhibitors for adjunctive therapy of metastatic melanoma. Of note, adverse events mostly included mild-to-moderate flu-like symptoms
Further reading on this topic from non-dermatologic literature:
mRNA vaccines — a new era in vaccinology
Melanoma Vaccines: Clinical Status and Immune Endpoints
And that is it!! Hope you did not get too much grey hair reading this article!!
Stay tuned for the next installment in this series coming up in January 2021!
Did you enjoy this article? You can find more here.