Keratoacanthomas are a low-grade, well-differentiated variant of squamous cell carcinoma. Characteristically, their abrupt-onset and crateriform microscopic findings assist with their differentiation from more aggressive squamous cell carcinoma. While surgical treatment remains a viable and appropriate option for their management, clinical scenarios (described below) may portend to better outcomes with intralesional therapy via methotrexate (MTX) or 5-fluorouracil (5-FU). Herein, we discuss the use of these intralesional therapies in the management of keratoacanthoma tumors.
Intralesional Methotrexate & 5-Fluorouracil for Keratoacanthomas
Cheat Sheet
Compiled By: Michael J. Visconti, DO | Reviewed By: Vishal A. Patel, MD
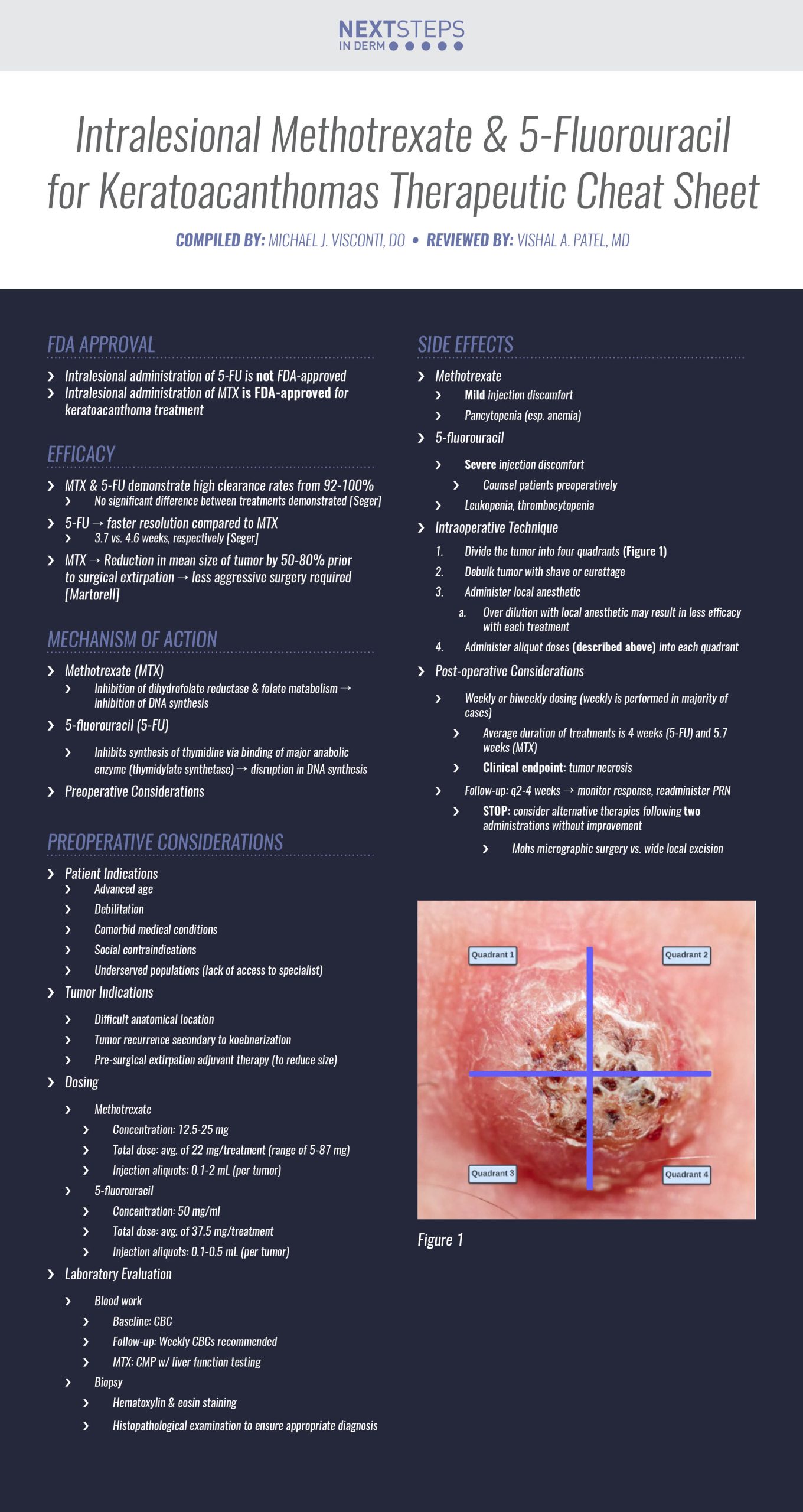
FDA Approval
-
- Intralesional administration of 5-FU is not FDA-approved
- Intralesional administration of MTX is FDA-approved for keratoacanthoma treatment
Efficacy
-
- MTX & 5-FU demonstrate high clearance rates from 92-100%
- No significant difference between treatments demonstrated [Seger]
- 5-FU → faster resolution compared to MTX
- 3.7 vs. 4.6 weeks, respectively [Seger]
- MTX → Reduction in mean size of tumor by 50-80% prior to surgical extirpation → less aggressive surgery required [Martorell]
- MTX & 5-FU demonstrate high clearance rates from 92-100%
Mechanism of Action
Methotrexate (MTX)
-
- Inhibition of dihydrofolate reductase & folate metabolism → inhibition of DNA synthesis
5-fluorouracil (5-FU)
-
- Inhibits synthesis of thymidine via binding of major anabolic enzyme (thymidylate synthetase) → disruption in DNA synthesis
Preoperative Considerations
Patient Indications
-
- Advanced age
- Debilitation
- Comorbid medical conditions
- Social contraindications
- Underserved populations (lack of access to specialist)
Tumor Indications
-
- Difficult anatomical location
- Tumor recurrence secondary to koebnerization
- Pre-surgical extirpation adjuvant therapy (to reduce size)
Dosing
-
- Methotrexate
- Concentration: 12.5-25 mg
- Total dose: avg. of 22 mg/treatment (range of 5-87 mg)
- Injection aliquots:
- 1-2 mL (per tumor)
- 5-fluorouracil
- Concentration: 50 mg/ml
- Total dose: of 37.5 mg/treatment
- Injection aliquots:
- 0.1-0.5 mL (per tumor)
- Methotrexate
Laboratory Evaluation
-
- Blood work
- Baseline: CBC
- Follow-up: Weekly CBCs recommended
- MTX: CMP w/ liver function testing
- Biopsy
- Hematoxylin & eosin staining
- Histopathological examination to ensure appropriate diagnosis
- Blood work
Side Effects
Methotrexate
-
- Mild injection discomfort
- Pancytopenia (esp. anemia)
5-fluorouracil
-
- Severe injection discomfort
- Counsel patients preoperatively
- Leukopenia, thrombocytopenia
- Severe injection discomfort
Intraoperative Technique
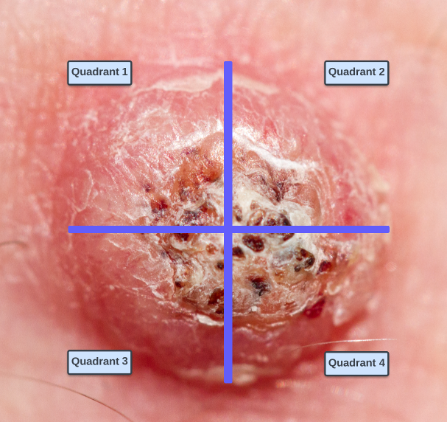
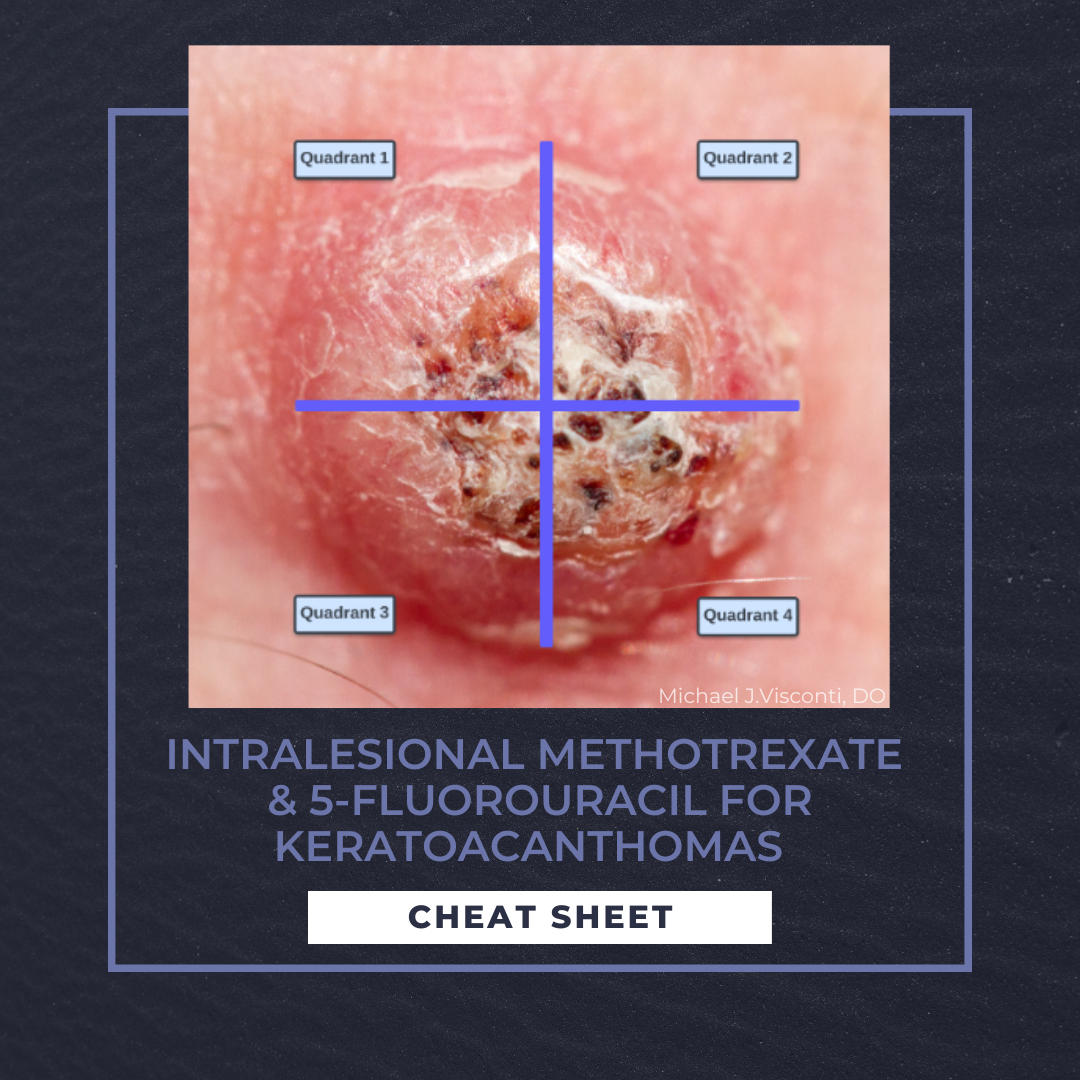
1. Divide the tumor into four quadrants (Figure 1)
2. Debulk tumor with shave or curettage
3. Administer local anesthetic
a. Over dilution with local anesthetic may result in less efficacy with each treatment
4. Administer aliquot doses (described above) into each quadrant
Post-operative Considerations
-
- Weekly or biweekly dosing (weekly is performed in majority of cases)
- Average duration of treatments is 4 weeks (5-FU) and 5.7 weeks (MTX)
- Clinical endpoint: tumor necrosis
- Follow-up: q2-4 weeks → monitor response, readminister PRN
- STOP: consider alternative therapies following two administrations without improvement
- Mohs micrographic surgery vs. wide local excision
- STOP: consider alternative therapies following two administrations without improvement
- Weekly or biweekly dosing (weekly is performed in majority of cases)
CLICK ON THE IMAGE BELOW TO ENLARGE AND/OR DOWNLOAD YOUR THERAPEUTIC CHEAT SHEET
-
- Annest, N. M., VanBeek, M. J., Arpey, C. J., & Whitaker, D. C. (2007). Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. Journal of the American Academy of Dermatology, 56(6), 989-993.
- Eubanks, S. W., Gentry, R. H., Patterson, J. W., & May, D. L. (1982). Treatment of multiple keratoacanthomas with intralesional fluorouracil. Journal of the American Academy of Dermatology, 7(1), 126-129.
- Kim, C. Y., Yoon, T. J., & Kim, T. H. (2001). Treatment of keratoacanthomas with intralesional 5-fluorouracil and methotrexate. Korean Journal of Dermatology, 39(10), 1175-1178.
- Leonard, A. L., & Hanke, C. W. (2006). Treatment of giant keratoacanthoma with intralesional 5-fluorouracil. Journal of Drugs in Dermatology: JDD, 5(5), 454-456.
- Martorell-Calatayud, A., Requena, C., Nagore, E., Sanmartín, O., Serra-Guillén, C., Botella-Estrada, R., … & Guillén-Barona, C. (2011). Intralesional infusion of methotrexate as neoadjuvant therapy improves the cosmetic and functional results of surgery to treat keratoacanthoma: results of a randomized trial. Actas Dermo-Sifiliográficas (English Edition), 102(8), 605-615.
- Odom, R. B., & Goette, D. K. (1978). Treatment of keratoacanthomas with intralesional fluorouracil. Archives of Dermatology, 114(12), 1779-1783.
- Seger, E. W., Tarantino, I. S., Neill, B. C., & Wang, T. (2020). Relative efficacy of nonoperative treatment of keratoacanthomas. Journal of Cutaneous Medicine and Surgery, 24(1), 41-46.
- Yoo, M. G., & Kim, I. H. (2014). Intralesional methotrexate for the treatment of keratoacanthoma: Retrospective study and review of the korean literature. Annals of dermatology, 26(2), 172-176.
Did you enjoy this post? Find more on Surgical Dermatology here.