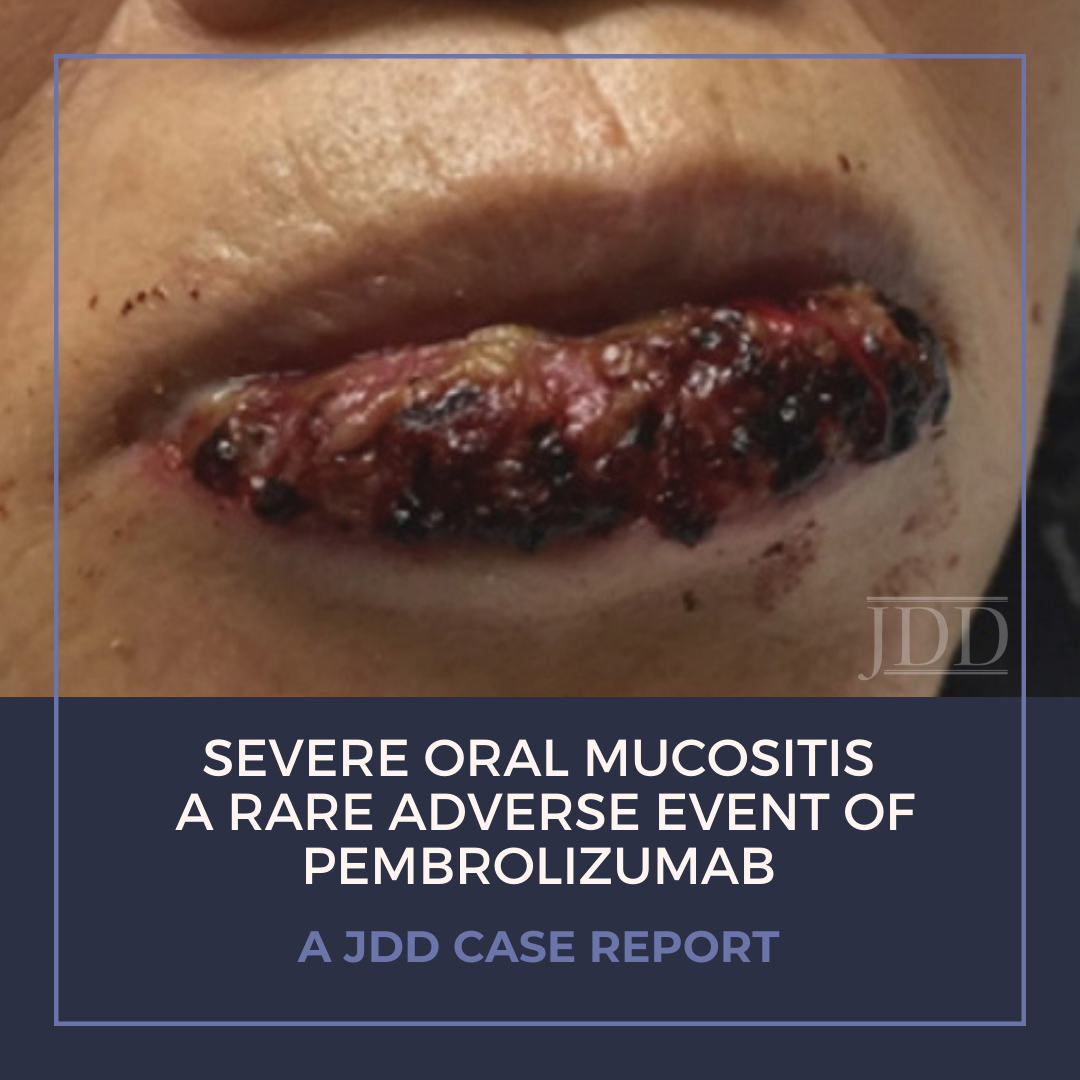
Treatment of malignancy with anti-programmed cell death 1 (PD-1) immune checkpoint inhibitors can cause mucocutaneous side effects resulting from T cell activation. Due to their recent development, the full side effect profile remains to be fully elucidated, however dermatologic adverse events are most common. The main oral toxicities of these immune checkpoint inhibitors include: xerostomia, dysgeusia, and lichenoid reactions. Oral mucositis occurs more rarely in the setting of PD-1 inhibition, and few other reports of a Grade 3 or higher, severe, stomatitis have been reported in the literature. JDD authors present a case of a 78-year-old woman with Grade 3 ulcerative oral mucositis that occurred 13 months after initiation of PD-1 inhibitor, pembrolizumab, for the treatment for lung adenocarcinoma. She was successfully treated with prednisone, and pembrolizumab was temporarily held by her oncologist. Physicians should be aware of the possibility of severe mucositis in the setting of PD-1 inhibitors, as well as the management.
Case Report
Discussion
Immune checkpoint inhibition is a novel treatment strategy intended to activate the immune system against malignancy.¹ Pembrolizumab is an IgG4 antagonist monoclonal antibody to programmed-cell-death-1 (PD-1) receptor approved by the FDA in 2014.1,2 When PD-1 binds to programmed-cell-death-ligand-1 (PD-L1), T-cell proliferation is suppressed; thus tumor cells that express PD-L1 are protected from cytotoxic T-cell-mediated tumor destruction.1 By preventing this suppression of host T-cells targeting tumor cells, PD-1 and PD-L1 inhibitors are being used in the treatment of multiple malignancies.1 However, PD-1 inhibitors also remove self-protective PD-1-mediated T-cell inhibition and thus have the potential to trigger autoimmune-related adverse events.2,3 While such autoimmune-related side-effects have been described, given the recent development of these immunotherapies, the extent of adverse events is still not well delineated.2 The most common adverse events reported with anti-PD-1 antibodies are cutaneous reactions, largely non-specific maculopapular skin eruptions, and pruritus; however, lichenoid dermatitis and mucosal involvement have been reported.3-5 Oral mucosal involvement has been described,2-4,6 although only one previous report of severe oral mucositis (Grade 3 or higher) has been reported.7
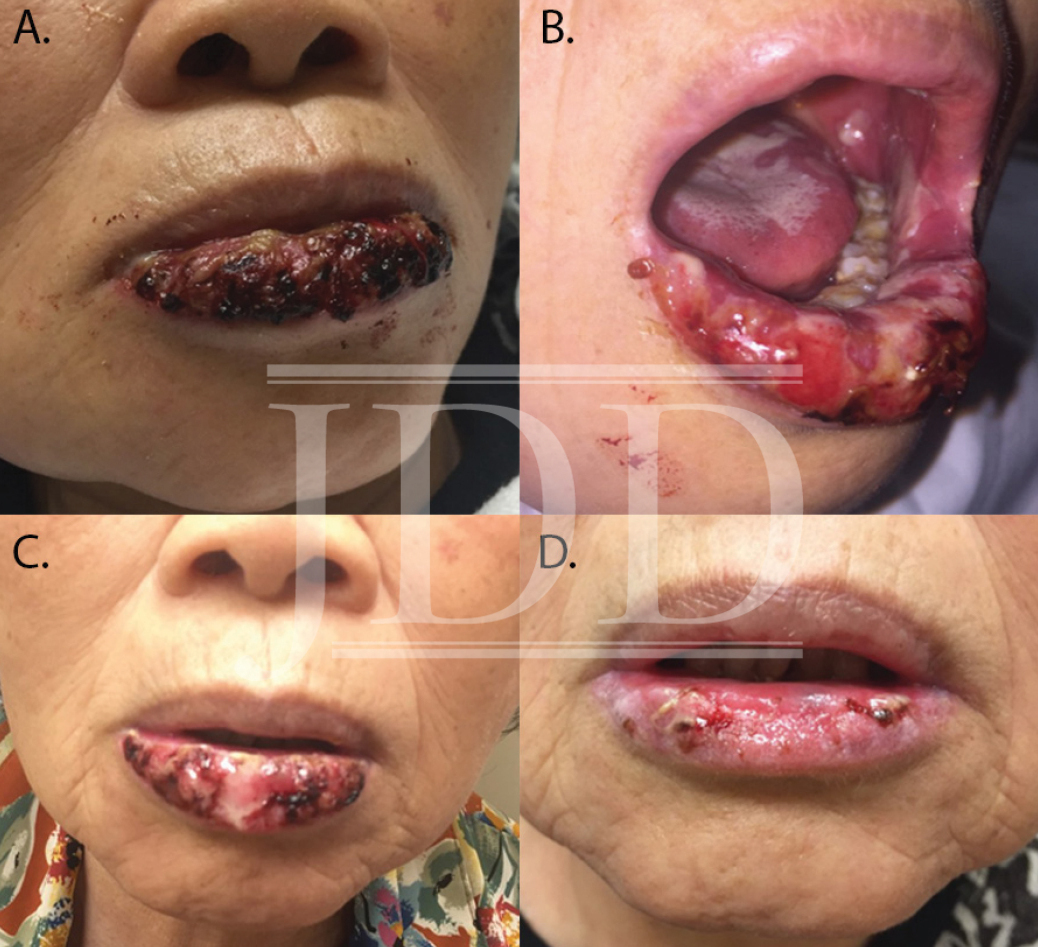
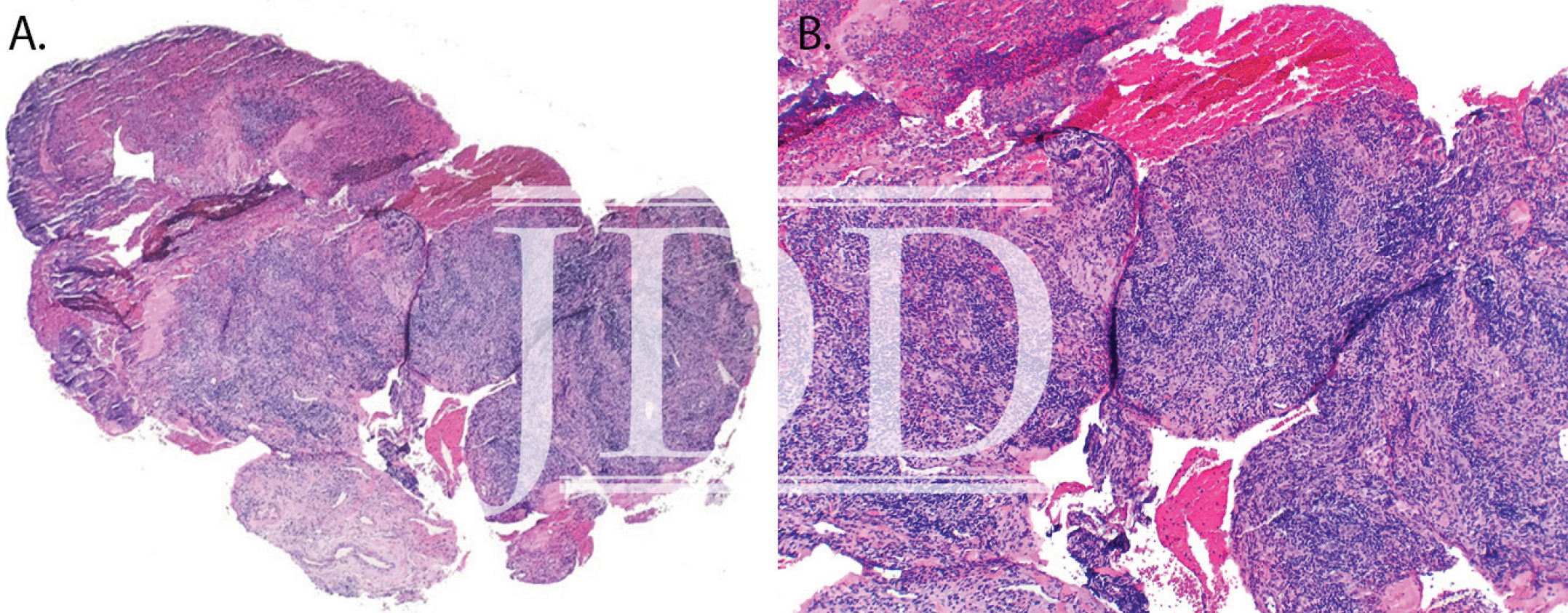
Here we present a case of ulcerative mucositis representing a severe Grade 3 reaction associated with PD-1 inhibitor pembrolizumab in a patient undergoing this therapy for treatment of lung adenocarcinoma. The differential diagnosis included paraneoplastic pemphigus and infectious etiologies such as HSV-associated mucositis and mycoplasma-pneumoniae-induced rash and mucositis (MIRM). Biopsy for DIF and IIF serologies were negative for paraneoplastic pemphigus. There was initial concern for MIRM versus HSV-associated mucositis due to the confounding positive IgM titers for both MP and HSV, although HSV PCR and viral culture were negative. Furthermore, her eruption persisted despite appropriate empiric treatment with acyclovir. MIRM was thought to be unlikely as it classically presents in children and adolescents, rarely involves fewer than two mucosal sites, and often has a prodrome of cough, fever, or malaise. Additionally, diagnosis should not rely on MP IgM titer, which may be elevated in patients without an acute infection8. Azithromycin was added to this patient’s course empirically, without effect, although MIRM typically resolves within weeks regardless of treatment.8
Our patient presented with delayed oral mucosal symptoms which began after 13 months of pembrolizumab therapy. The onset of cutaneous adverse events with PD-1 inhibitors has been described as late as 15 months after initial treatment, and incidence appears to rise with increasing length of treatment time.9,10 A recent case series demonstrated that although the clinical presentation of anti-PD-1 mucocutaneous adverse effects were varied, 94% of patients’ biopsies were demonstrative of a lichenoid interface dermatitis.2 In this case, lack of epidermis on histopathology prevented assessment for lichenoid features. Oral lichenoid eruptions in the setting of PD-1/PD-L1 inhibitors often presents as confluent white papules or reticular streaks, consistent with Wickham’s striae, which may involve the dorsal or lateral sides of the lips, tongue, gingiva, hard palate or buccal mucosae.2,4,6 The affected areas may be painful or asymptomatic.3,4,6 Infrequent cases of oral mucositis have been reported.
The majority of PD-1-inhibitor-induced mucocutaneous reactions are self-limiting.2-4 In the presence of painful oral lesions, treatment with topical steroids and dexamethasone swish and spit solution have been reportedly effective.2-4,6 One other reported case of severe mucositis occurred in a patient who had undergone 14 cycles of pembrolizumab for the treatment of laryngeal squamous cell carcinoma; pembrolizumab was discontinued and the patient was treated with IV methylprednisolone 2 mg/kg/day followed by a slow taper of oral prednisone over the course of 5 months due to recurrence.7 However, discontinuation of therapy is rarely necessary, as most reactions are not severe enough to warrant such management.2-4 Our patient was initially recalcitrant to oral corticosteroids, most likely because of the continued treatment with a PD-1 inhibitor. However, continued treatment with oral corticosteroids after pembrolizumab was held led to near resolution of mucositis at six-week follow-up. In the case of severe mucosal reactions, temporary cessation of the PD-1 inhibitor may be considered; however, given treatment of the underlying malignancy is of utmost priority, if it is not feasible to withhold PD-1 inhibitor, initiation of elevated dosage of corticosteroids, such as that described by Acero et al, may be beneficial.7 As the use of immunotherapy is increasing, providers should be aware of the potentially severe oral mucosal adverse reactions as well as their management.
References
-
- Wang X, Huang S, Zhang Y, et al. The application and mechanism of PD pathway blockade for cancer therapy. Postgrad Med J. 2017.
- Shi VJ, Rodic N, Gettinger S, et al. Clinical and Histologic Features of Lichenoid Mucocutaneous Eruptions Due to Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Immunotherapy. JAMA Dermatol. 2016;152(10):1128-1136.
- Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28(4):254-263.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol. 2015;151(11):1206-1212.
- Vigarios E, Epstein JB, Sibaud V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer. 2017;25(5):1713-1739.
- Acero Brand FZ, Suter N, Adam JP, et al. Severe immune mucositis and esophagitis in metastatic squamous carcinoma of the larynx associated with pembrolizumab. J Immunother Cancer. 2018;6(1):22.
- Vujic I, Shroff A, Grzelka M, et al. Mycoplasma pneumoniae-associated mucositis–case report and systematic review of literature. J Eur Acad Dermatol Venereol. 2015;29(3):595-598.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J Am Acad Dermatol. 2016;74(3):455-461 e451.
- Goldinger SM, Stieger P, Meier B, et al. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin Cancer Res. 2016;22(16):4023-4029.
Margo H. Lederhandler MD, Anthony Ho BA, Nooshin Brinster MD, Roger S. Ho MD, Tracey N. Liebman MD, Kristen Lo Sicco MD, (2018). Severe Oral Mucositis: A Rare Adverse Event of Pembrolizumab. Journal of Drugs in Dermatology, 17(7), 807-809.
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.
Did you enjoy this case report? Find more here.