INTRODUCTION
CASE
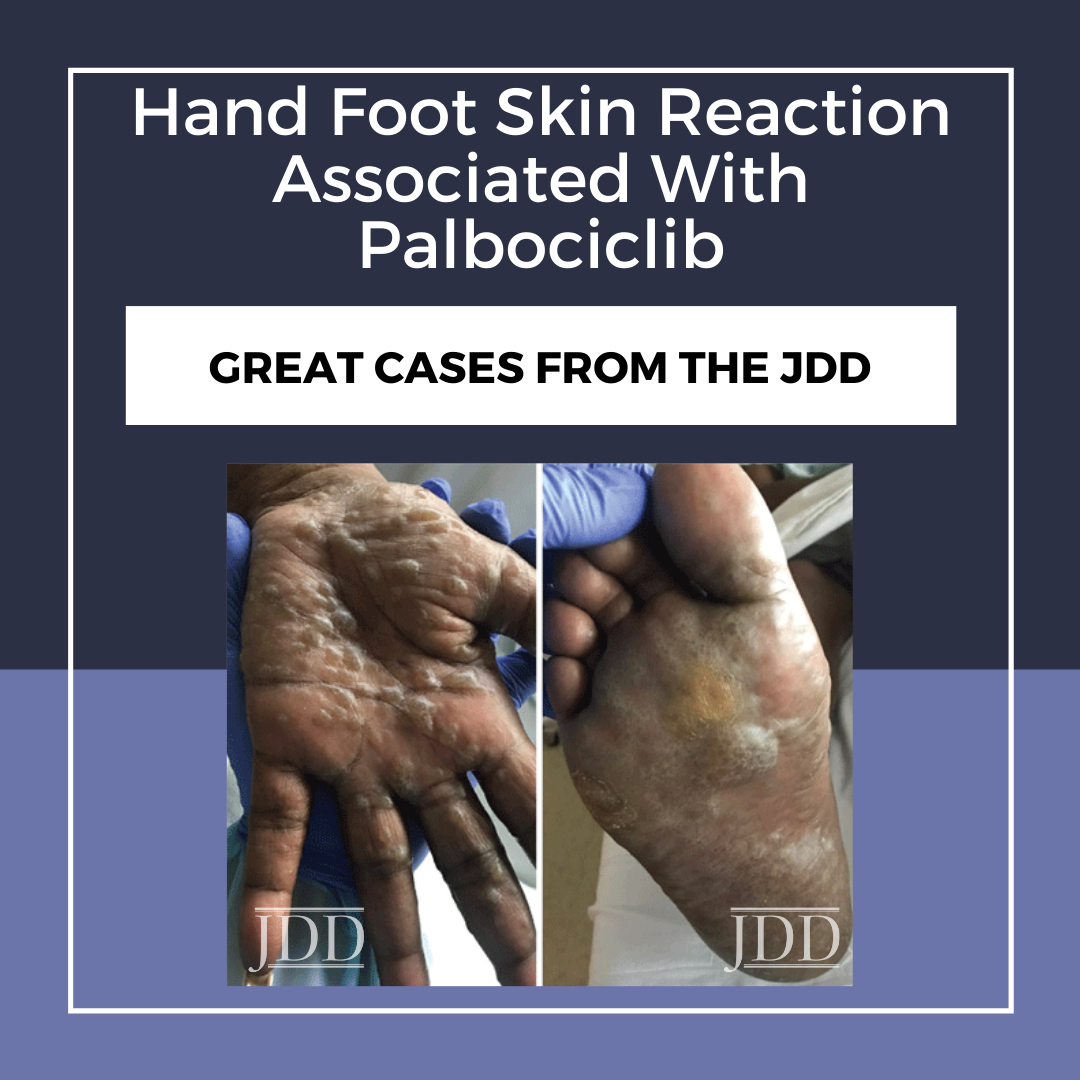
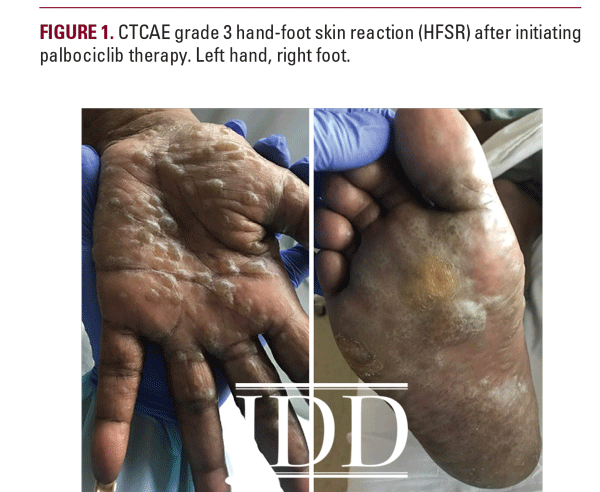
A 57-year-old African American woman presented with a 2-week history of painful blisters progressing to hyperkeratotic plaques on her palms and soles 6 weeks after initiation of palbociclib 125mg once a day for stage IIIA HR+Her2- breast cancer. Her other medications included fulvestrant. Previous therapies had included mastectomy and lymph node excision followed by chemotherapy (paclitaxel, doxorubicin, and cyclophosphamide), radiation, and anastrozole. She had no personal history of cutaneous disorders. Physical examination revealed multifocal hyperkeratotic plaques on the palms and soles with severe associated tenderness (Figure 1). The clinical characteristics of this patient’s presentation were consistent with HFSR due to targeted chemotherapy, which typically presents with hyperkeratotic papules or plaques on the hands and feet, dry and/or cracked skin, callous-like dry blisters, dysesthesia, and paresthesia.4 Given her severe skin changes and extreme pain limiting activities of daily living, she was diagnosed with grade 3 HFSR using the Common Terminology Criteria for Adverse Events (CTCAE) scoring system, version 5.0.5 Hand-foot syndrome appeared unlikely due to the lack of erythema or desquamation.
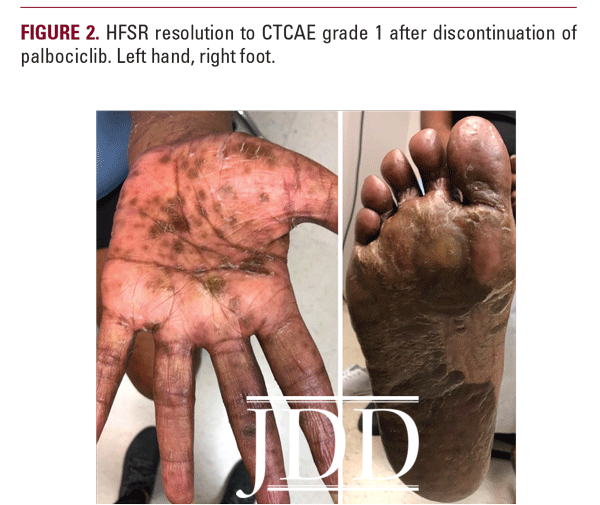
Due to the severity of her HFSR, palbociclib was discontinued and the patient was treated with urea 40% cream BID, clobetasol 0.05% ointment BID, and oral prednisone 60mg daily for 1 week. Two weeks after discontinuation of palbociclib, her rash had improved to CTCAE grade 1 with no new lesions, interval improvement in previous tenderness and hyperkeratosis, and associated desquamation (Figure 2). The patient subsequently developed cutaneous metastastic lesions from her breast cancer 1 month after discontinuing palbociclib and was started on alpelisib.
DISCUSSION
The temporal course of the HFSR suggests a palbociclibinduced etiology. The skin changes appeared a few weeks after initiating palbociclib and resolved rapidly after discontinuation, which is consistent with other reports of drug-induced HFSR. For example, in the setting of tyrosine kinase inhibitor (TKI) induced HFSR, the skin changes typically appear during the first month of treatment and remit within weeks of discontinuing therapy.4,6 The patient was not on other medications that could have caused the HFSR.
Drug induced HFSR is a well described side effect of selective Raf inhibitors as well as multikinase inhibitors that target Raf. Interestingly, Raf inhibition has been shown to upregulate expression of endogenous CDK inhibitors (p16, p27, p57).7Thus, there may be a mechanistic link between Raf-inhibition-induced and CDK4/6-inhibitor-induced HFSR, though this relationship remains undefined and warrants further study. The currently accepted theory for the pathogenesis of HFSR is that of dermal capillary micro-trauma leading to drug extravasation, tissue damage, and inhibition of vascular repair at sites prone to mechanical and frictional stress.8
Physicians should be aware of this potential side effect of palbociclib as early consultation with dermatology and early intervention may avoid the need for dose interruption of lifesustaining oncologic therapy.9 Treatment centers around symptomatic therapy with urea-containing keratolytic emollients followed by high-potency topical steroids or oral corticosteroids in severe cases.4,6,8 Patients should also avoid tight-fitting shoes, use shock absorbers, and keep skin well moisturized.4,6,8 For cases requiring dose interruption or discontinuation, whether palbociclib can be reinitiated without recurrence of HFSR remains to be determined.
DISCLOSURES
REFERENCES
2. Guo L, Hu Y, Chen X, et al. Safety and efficacy profile of cyclin-dependent kinases 4/6 inhibitor palbociclib in cancer therapy: A meta-analysis of clinical trials. Cancer Med. 2019;8(4):1389-1400.
3. Khan, NAJ, Alsharedi, M. Bullous skin rash: a rare case of palbociclib-induced dermatological toxicity. Cureus.2020;12(9): e10229.
4. Porta, C, Paglino, C, Imarisio, I, Bonomi, L. Uncovering Pandora’s vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med. 2007;7:127-134.
5. Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: November 27. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute.
6. Autier, J, Escudier, B, Wechsler, J, et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144(7):886-892.
7. Curry, JL, Falchook, GS, Hwu, W, et al. Changes in tumor morphology and cyclin-dependent kinase inhibitor expression in metastatic melanoma treated with selective second-generation BRAF inhibitor. Am J Dermatopathol. 2013;35:125-128.
8. Miller, KK, Gorcey, L, McLellan, BN. Chemotherapy-induced hand-foot skin syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71(4):787.
9. Barrio, D, Ciccolini, D, Phillips, G, et al. Anticancer therapy interruption and diagnostic concordance between referring clinicians and dermatologists at Memorial Sloan Kettering Cancern Center. J Am Acad Dermatol. 2017;76(6):AB45.
SOURCE
Lee, V., & Chadha, A. A. Hand Foot Skin Reaction Associated With Palbociclib. Journal of drugs in dermatology: JDD, 20(7). Published early online June 17 2021.
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.
Did you enjoy this case report? Find more here.