INTRODUCTION
Tofacitinib is a Janus kinase (JAK) 1-3 inhibitor first U.S. Food and Drug Administration (FDA) approved in 2012 for rheumatoid arthritis, with subsequent approval for psoriatic arthritis, ulcerative colitis, polyarticular course juvenile idiopathic arthritis, and ankylosing spondylitis in 2017, 2018, 2020, and 2021, respectively.1,2 In the last several years, oral tofacitinib has become an increasingly utilized treatment option for immune-mediated skin conditions such as alopecia areata (AA), vitiligo, plaque psoriasis, and atopic dermatitis; however, it is not FDA approved for these diseases.3,4 These conditions’ pathophysiology involves an interplay of cytokines and interleukins in which the JAK-signal transducer and activation of transcription (STAT) signaling pathway has a role.5
AA and vitiligo are largely TH1 and interferon-gamma driven diseases.6 In AA, CD8+ T cells produce interferon-gamma which leads to collapse of immune privilege and increased production of IL-15, propagating autoimmune destruction of the hair follicle.6 In vitiligo, an autoimmune disease in which melanocytes are destroyed resulting in de-pigmentation, interferon-gamma induces expression of the C-X-C motif in chemokine-10 (CXCL10) in immune cells, leading to melanocyte destruction and depigmentation.7 Notably, interferon-gamma mediates its effects through the JAK-STAT pathway.5,8 Due to the relationship between AA and vitiligo with interferon gamma and the JAK-STAT pathway, JAK inhibitors, such as tofacitinib, are an appropriate choice for targeted therapy of these conditions. In this case report, we present a patient with concomitant AA and non-segmental vitiligo who was treated with oral tofacitinib. The autoimmune components of both diseases and similar pathophysiology cause AA and vitiligo to occur together more often than one would expect by chance; with approximately 3-8% of patients with AA having concomitant vitiligo.9 The patient in this report attempted multiple different treatment regimens with minimal improvement, however, upon initiation with tofacitinib, had moderate to significant improvement in both her AA and vitiligo, with no reported side effects.
CASE REPORT
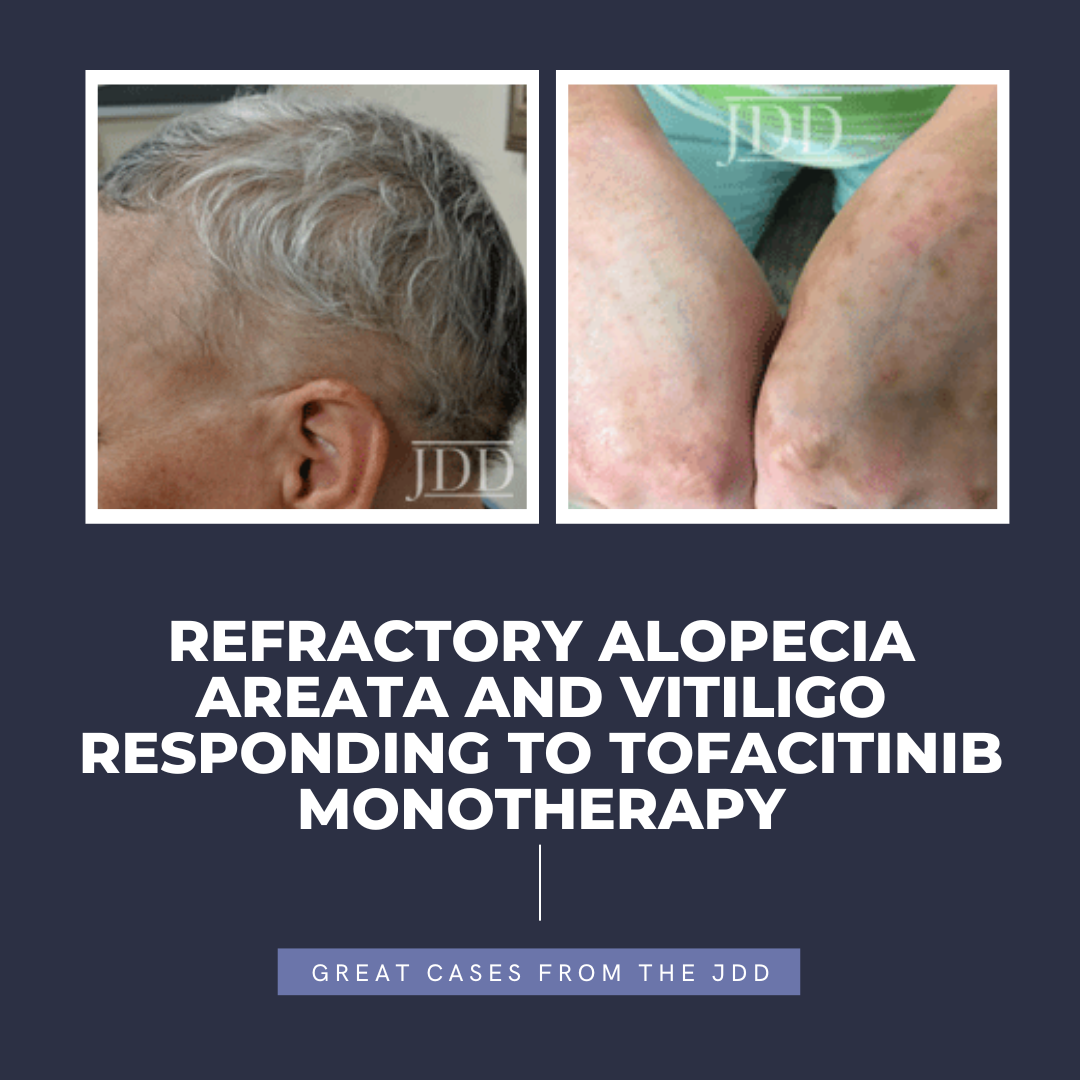
tofacitinib.

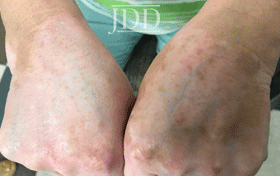
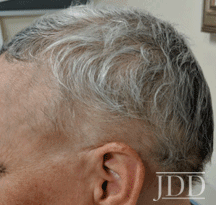
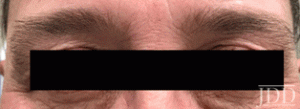
with tofacitinib illustrating re-pigmentation.
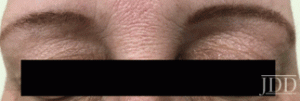
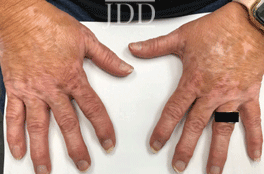
with tofacitinib.
DISCUSSION
Only two case reports exist in the literature of patients with concomitant alopecia areata and vitiligo who were treated with tofacitinib.1,3 Of those cases, one patient additionally had inverse plaque psoriasis, while the other patient additionally had atopic dermatitis. In both of these cases, tofacitinib was effective; however, it was utilized as adjunctive therapy rather than monotherapy. In our case report, we present a patient treated with low-dose tofacitinib monotherapy. Our patient’s response to tofacitinib monotherapy is similar to that of other patients on multiple treatments, where clinical response was observed as quickly as 2 months with significant improvement reached around 6-8 months of treatment.1
Tofacitinib is well studied in AA with improvement rates of >50% in 30-60% of patients on tofacitinib 5 mg BID.10-12 In vitiligo, the use of tofacitinib is less studied with some patients reporting marginal improvement and others reporting near complete repigmentation.1,7,13-16 In these reports, tofacitinib is commonly used concordantly with ultraviolet B phototherapy. In general, patients have greater improvement in areas of sun exposure or those additionally treated with phototherapy. Nonetheless, our patient had good improvement in her hand vitiligo, with minimal reported sun exposure and no additional phototherapy.
The use of JAK inhibitors off-label has become increasingly utilized and is effective for the treatment of AA, vitiligo, and other immune-mediated diseases. While JAK inhibitors are associated with several Black Box warnings and require careful monitoring, they may be a suitable alternative for patients refractory to previous treatments. In particular, in the few patients in the literature who have concomitant AA and vitiligo, tofacitinib appears to be an effective treatment option.
DISCLOSURES
REFERENCES
2. Pfizer. Xeljanz (Tofacitinib) [package insert]. U.S. Food and Drug Administration website. www.accessdata.fda.gov/drugsatfda_docs/lab el/2021/203214s028,208246s013,213082s003lbl.pdf#page=66. Revised December 2021. Accessed December 16, 2021.
3. Tajalli M, Kabir S, Vance TM, et al. Effective use of oral tofacitinib and phototherapy in a patient with concomitant alopecia areata, vitiligo, and plaque and inverse psoriasis. Clin Case Rep. 2020;8(5):819-822. doi:10.1002/ ccr3.2759
4. Shreberk-Hassidim R, Ramot Y, Zlotogorski A. Janus kinase inhibitors in dermatology: A systematic review. J Am Acad Dermatol. 2017;76(4):745-753. e19. doi:10.1016/j.jaad.2016.12.004
5. Damsky W, King BA. JAK inhibitors in dermatology: The promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736-744. doi:10.1016/j. jaad.2016.12.005
6. Harris JE. Vitiligo and alopecia areata: apples and oranges? Exp Dermatol. 2013;22(12):785-9. doi:10.1111/exd.12264
7. Craiglow BG, King BA. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 2015;151(10):1110-2. doi:10.1001/jamadermatol.2015.1520
8. Horvath CM. The Jak-STAT pathway stimulated by interferon gamma. Sci STKE. 2004;2004(260):tr8. doi:10.1126/stke.2602004tr8
9. Rork JF, Rashighi M, Harris JE. Understanding autoimmunity of vitiligo and alopecia areata. Curr Opin Pediatr. 2016;28(4):463-469. doi:10.1097/ MOP.0000000000000375
10. Ibrahim O, Bayart CB, Hogan S, et al. Treatment of alopecia areata with tofacitinib. JAMA Dermatol. 2017;153(6):600-602. doi:10.1001/ jamadermatol.2017.0001
11. Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: A study of 90 patients. J Am Acad Dermatol. 2017;76(1):22-28. doi:10.1016/j.jaad.2016.09.007
12. Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. doi:10.1172/jci.insight.89776
13. Joshipura D, Plotnikova N, Goldminz A, et al. Importance of light in the treatment of vitiligo with JAK-inhibitors. J Dermatolog Treat. 2018;29(1):98- 99. doi:10.1080/09546634.2017.1339013
14. Gianfaldoni S, Tchernev G, Wollina U, et al. Micro – Focused phototherapy associated to janus kinase inhibitor: a promising valid therapeutic option for patients with localized vitiligo. Open Access Maced J Med Sci. 2018;6(1):46- 48. doi:10.3889/oamjms.2018.042
15. Liu LY, Strassner JP, Refat MA, et al. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77(4):675-682.e1. doi:10.1016/j.jaad.2017.05.043
16. Relke N, Gooderham M. The Use of Janus Kinase Inhibitors in Vitiligo: A Review of the Literature. J Cutan Med Surg. 2019;23(3):298-306. doi:10.1177/1203475419833609
17. Ahn CS, Culp L, Huang WW, et al. Adherence in dermatology. J Dermatolog Treat. 2017;28(2):94-103. doi:10.1080/09546634.2016.1181256
SOURCE
Content and images used with permission from the Journal of Drugs in Dermatology.
Adapted from original article for length and style.